Rejuvenation biotechnology encompasses a suite of advanced medical therapies, each of which removes, repairs, replaces, or renders harmless one of the forms of cellular or molecular damage that accumulates in an aging tissue over time and impairs its function. Through the comprehensive abatement of all such aging damage to levels approximating those of younger adults, tissue structure and function can be made more youthful, restoring the health and vigor of aging persons to that of persons years or decades younger. This approach is most prominently under pursuit in the development of cell therapy and tissue engineering, of which the most striking success to date has been the use of fetal and embryonic mesencephalic tissue grafts to replace dopaminergic (DA) neurons lost to the age-related neurodegenerative processes driving Parkinson’s disease (PD).(0)
The most immediate cause of the loss of fine motor control that leads to the most prominent motion disorders characteristic of the disease is the local loss of DA release by substantia nigra pars compacta (SNc) DA neurons into their striatal targets. In studies to date, DA cells have been transplanted directly into the striatum, where they reinnervate and partly functionally integrate the tissue, locally restoring the needed release of DA that is essential to fine motor control.
While such grafts have led to striking temporary improvements in the major motion disorder symptoms in many patients, results have been limited, and significant improvements in the protocol are clearly required to realize the potential of cell therapy to help these patients. The grafts are significantly immunogenic, leading to rejection in some cases and necessitating immunosuppression in most patients. The nature of the cell source means that very few patients could ever hope to benefit from treatment, since the supply of fetal midbrain tissue is both inherently limited and subject to politically-imposed restrictions. And the actual benefits to patients have not been as robust as might have been hoped: the magnitude of clinical response has been highly variable, gains have proven impermanent, and ~15% of transplanted patients have developed substantial dyskinesias during the “off” phase of levodopa treatment.(0)
Many of these limitations are attributable to the crude cell sources used in these trials. An important step forward will be to move beyond the use of fetal mesencephalic tissue, and instead derive graft populations of pure DA neurons from pluripotent stem cells, such as embryonic stem cells (ESC), induced pluripotent stem cells (iPS), or somatic cell nuclear transfer (SCNT — “therapeutic cloning”). Any of these sources should substantially alleviate the problem of immunogenicity of fetal tissue: ESC-derived differentiated cells and progenitors appear to enjoy some degree of immunological privilege,(1) and iPS- or SCNT-derived cells would be immunologically native and free of rejection risk, with the possible exception of mitochondrially-derived immunogenicity in SCNT. As well, the graft-induced dyskinesias now appear to be largely attributable to the presence of serotonergic neurons in the mixed cell population present in the graft tissue,(2) which would not be present with DA cells specifically derived from pluripotent stem cells. And the nearly-unlimited replicative capacity of pluripotent cells, combined with an efficient and stable means of differentiation, would provide such cells in the quantities needed for widespread clinical use in frank PD and in “normative” brain aging.
The promise of this approach has been foreshadowed in murine models of PD, in which DA neurons derived from mouse ESC have been found highly effective in reversing motor symptoms. But the performance of ostensibly DA neurons derived from human pluripotent stem cells in the same systems has so far been poor, due to uncertain and unstable differentiation of the cells. In a new study,(3) a team of researchers led by Dr. Lorenz Studer of the Sloan-Kettering Institute’s Center for Cell Engineering have used their novel DA neuron differentiation strategy to resolve these difficulties, leading to robust and stable engraftment of human pluripotent stem cell-derived DA neurons into the striatum and substantial evidence of efficacy in two rodent models of the disease, and provided preliminary data on the viability of their approach in nonhuman primates.
A Superior Protocol
The authors had previously reported(4) a protocol for deriving mesencephalic DA neurons by nudging them through an intermediary stage as midbrain floor plate precursor cells. The floor plate is the developmental topos through which cells are thought to acquire DA neural progenitor characteristics. This entailed a dual inhibition of SMAD signalling in the cells through the simultaneous inhibition of BMP4 using Noggin (an inhibitor of BMP4), and activation of the Lefty/Activin/TGFβ pathway with the drug SB431542, and the approach has subsequently been independently validated by investigators at the MRC Centre for Regenerative Medicine.(5) As part of this new report,(3) two lines each of human ESC and iPS cells were used to derive DA neurons using either a modified version of this new strategy, or the currently-standard protocol(6) of moving such cells through a neural rosette intermediate for DA neuron derivation. The dual SMAD inhibition, floor-plate-intermediate protocol proved superior, generating a higher percentage of tyrosine hydroxylase-expressing (TH+) neurons, which unlike rosette-derived cells expressed markers of the developing mesencephalon and (importantly) led to far lower adventitious generation of serotonergic neurons.(3)
Dopaminergic Phenotype
One weakness common to many studies of derivation of differentiated cells from pluripotent precursors has been the almost exclusive reliance on cell-surface markers as indicators of ultimate fate, casting uncertainty over the nature of such cells and the correct interpretation of their observed therapeutic benefits, limitations, and adverse reactions in models of injury and disease. The authors loaned substantial credibility to their results by showing that their human ESC- and iPS-derived DA cells recapitulate multiple phenotypic characteristics of native SNc DA neurons and of SNc neurons grown from early postnatal mice, including not only high expression of their mature neuronal markers, but their characteristic electrophysiological phenotype and extensive fiber outgrowth.(3)
Success and Safety In Vivo
The investigators next performed in vivo tests of cells derived either from their own modified dual SMAD-inhibition protocol, or using the rosette method. Based on previous work, the investigators performed all transplantation when the therapeutic cells were in cell cycle exit; such cells proved to survive effectively in intact rodents, and were next tested by striatal injection into brains of mice whose SNc DA neurons had been unilaterally destroyed using 6-hydroxy-dopamine (6-OHDA), a standard model of PD.(3) Because it has proven challenging to develop differentiated cells that exhibit robust growth and strong engraftment in local neuronal tissue without leading to either “off-target” differentiation into undesired cell types, or tumorigenic overgrowth, the researchers opted to perform these tests using the radically immunodeficient NOD-SCID IL2Rgc null mouse, which “efficiently supports xenograft survival with particular sensitivity for exposing rare tumorigenic cells.”(3) Moreover, neuronal cultures were grafted “as is” rather than following additional cell purification, to give maximum opportunity for any insufficiently- or improperly-differentiated cells to reveal themselves in overgrowth.
At four and a half months post-transplant, the fates of cells derived from the two protocols were dramatically different in vivo. In animals engrafted with DA neurons derived using the standard rosette method, administration of amphetamine led to stereotypic circling motions, caused by the drug’s stimulation of the surviving DA neurons remaining on the unlesioned side of the brain. By contrast, engraftment with cells derived using the floor plate method rescued this behavior. Similarly, in animals treated with rosette-derived DA neurons, there were few human-derived DA neurons present at the graft site, which was however riddled with proliferating Ki-671+ cells, and exhibited “massive neuronal overgrowth.”(3) The equivalent site in animals receiving floor plate-derived cells formed a distinctive hub, well-populated with human-derived DA neurons, and with <1% of total cells actively proliferating.(3)
To rule out any artifacts of their reliance on the immunodeficient mouse model, these experiments were repeated in rats immunosuppressed using cyclosporin A, this time comparing neural grafts using cells derived with their new protocol to sham surgery. Again, high numbers of transplanted DA neurons engrafted, survived, and branched out to integrate with surviving tissue without overgrowth; gene expression profiles suggested the presence of both nigral and ventral tegmental-area DA neurons. Treatment again eliminated rotational behavior following amphetamine administration, and also alleviated deficits in two tests of motor control that do not rely on drug-induced overactivation of surviving DA neurons: the stepping test of forelimb akinesia, and the specific use of the forelimb using the cylinder test.(3) In this model too, <1% of total cells had improperly differentiated into serotonergic neurons, and the few GFAP1 glial cells were host-derived; moreover, transplantation of their human pluripotent cell-derived DA neurons eliminated or improved each of the model motor disorders tested (see Figure 1).(3)
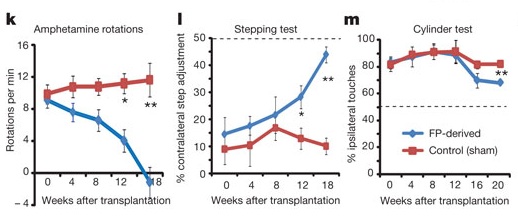
Figure 1. Floor-Plate (FP)-Derived Dopaminergic Neuron Grafts from Human ESC Rescue Motion Disorders in 6-OHDA-Lesioned Rats. Reproduced from (3).
Preliminary Promise in Primates
Finally, the investigators performed a minimal test the viability of their approach in a nonhuman primate model. In addition to being more closely related to our species, these animals’ lesioned brains can acommodate the far larger numbers of cells than those of the much smaller rodent models, better reflecting what would be needed in actual human cell therapy. Their system allowed for ready derivation of 5 x 107 DA neurons for testing, and following MPTP lesioning of the substantia nigra, each of two adult Rhesus monkeys received a total of six transplants of 1.25 x 106 cells, injected into each of three sites spanning the posterior caudate and pre-commissural putamen on each side of the brain. One month later, GFP expression revealed high numbers of surviving mesencephalic DA neurons at each site, which coexpressed the human-specific cytoplasmic marker SC-121; these cells’ fibers branched out of the graft cores for up to 3mm into surrounding host brain tissue. The sole note of caution was the suggestion of incomplete immunosuppression, based on the presence of Iba1+ host microglial cells in the grafts.(3)
Continuing, Perfecting and Integrating a Rejuvenation Biotechnology
As the researchers conclude, their
novel [floor plate]-based [pluripotent stem cell] differentiation protocol faithfully recapitulates midbrain DA neuron development. … Importantly, our study establishes a means of obtaining a scalable source of … neurons for neural transplantation — a major step on the road towards considering a cell based therapy for Parkinson’s disease. Excellent DA neuron survival, function, and lack of neural overgrowth in the three animal models indicate promise for the development of cell-based therapies in Parkinson’s disease.(3)
The most immediate next step in development of this potential therapy is to test the ability of these grafts to rescue the motion disorders of MPTP-lesioned nonhuman primates. Were such cells to prove themselves in this model, clinical trials in humans suffering with the crippling motion disorders of PD would be fully justified in light of the limited positive results already achieved in trials of cell therapy for this disorder. Engraftment with pure populations of pristine DA neurons should deliver far greater benefits than those previously observed using mixed fetal/embryonic tissue, reducing or eliminating the need for immunosuppressive thearapy and also minimizing or abrogating the dyskinesic side-effects that were apparently the result of contamination with serotonergic cells.(2) And, once proven safe and effective, the replicative capacity of pluripotent stem cells should allow such benefits to be delivered to far more patients.
Once these cells have proven their potential in humans, yet further refinement of the protocol can be expected to more fully alleviate the symptoms of PD. In this study,(3) as in the previous human(0) and preclinical work, transplanted cells have been injected directly into the striatum, where the loss of DA is acute. But native SNc DA neurons release DA not only at their striatal target sites, but throughout their soma and dendrites within the SNc itself; to fully restore the intact circuitry of the youthful, fully-functioning dopaminergic system will require protocols for the orthotopic transplantation of such cells into the neurodegenerative substantia nigra, recapitulating the physiological dopaminergic innervation of the striatum from this graft core. Fortunately, nonhuman primate work toward this goal is already underway.(10) The perfection of such protocols will enable us to move beyond even treatment of clinical PD, and into repair of the SNc and other DA neuronal structures lost during prodromal stages of the disease, and ultimately toward alleviating even the more subtle motion dysfunction caused by SNc DA neuronal losses suffered during “normal” brain aging.
Yet further gains will depend on combining this single rejuvenation biotechnology into a wider range of therapies to repair the agign brain. In the earlier trials, transplanted cells subsequently developed intraneuronal aggregates composed of α-synuclein, characteristic of the aging and particularly the PD brain, at a rate that appears to be modest as relates to graft survival times but perhaps faster than that in native, undiseased neurons.(7) The appearance of such damage in relatively recently-engrafted neurons contrasts with the temporal neuropathological staging of such pathology in the aging hindbrain, where it first appears in the lower brainstem, remote from the SNc and striatum, and gradually spreads forward toward it over time. There is strong evidence that the accumulation of such aggregates in extranigral sites plays a key role in PD, although its relationship with DA neuronal death is unclear and may well be independent; it is more likely that this more widespread damage underlies many of the nondopaminergic, levodopa-refractory symptoms of PD as a multisystem disorder.(8) Full brain rejuvenation will require the use of novel xenohydrolases(9) to clear aging neurons of these aggregates, both to maintain the benefits of transplanted cells, and to eliminate the troublesome and disabling symptoms that arise from aging damage to structures beyond the SNc.
A key barrier to the use of human pluripotent stem cells to rejuvenate the aging and neurodegenerative brain appears to have been broken. It is now our task to press on, treating Parkinson’s disease and ultimately ending the age-related degeneration of the human brain.
References
0: Lindvall O, Björklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004 Oct;1(4):382-93. Review. PubMed PMID: 15717042; PubMed Central PMCID: PMC534947.
1: English K, Wood KJ. Immunogenicity of embryonic stem cell-derived progenitors after transplantation. Curr Opin Organ Transplant. 2010 Dec 9. [Epub ahead of print] PubMed PMID: 21150615.
2: Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, Bjorklund A, Lindvall O, Piccini P. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med. 2010 Jun 30;2(38):38ra46. PubMed PMID: 20592420.
3: Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011 Nov 6. doi: 10.1038/nature10648. [Epub ahead of print] PubMed PMID: 22056989.
4: Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009 Mar;27(3):275-80. Epub 2009 Mar 1. Erratum in: Nat Biotechnol. 2009 May;27(5):485. PubMed PMID: 19252484; PubMed Central PMCID: PMC2756723.
5: Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M, Drummond NJ, Taanman JW, Schapira AH, Gwinn K, Hardy J, Lewis PA, Kunath T. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2011 Aug 23;2:440. doi: 10.1038/ncomms1453. PubMed PMID: 21863007.
6: Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008 Jan 15;22(2):152-65. Erratum in: Genes Dev. 2008 May 1;22(9):1257. PubMed PMID: 18198334; PubMed Central PMCID: PMC2192751.
7: Braak H, Del Tredici K. Assessing fetal nerve cell grafts in Parkinson’s disease. Nat Med. 2008 May;14(5):483-5. PubMed PMID: 18463652.
8: Lang AE, Obeso JA. Challenges in Parkinson’s disease: restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol. 2004 May;3(5):309-16. Review. PubMed PMID: 15099546.
9: Mathieu JM, Schloendorn J, Rittmann BE, Alvarez PJ. Medical bioremediation of age-related diseases. Microb Cell Fact. 2009 Apr 9;8:21. PubMed PMID: 19358742; PubMed Central PMCID: PMC2674406.
10: Redmond DE Jr, Weiss S, Elsworth JD, Roth RH, Wakeman DR, Bjugstad KB, Collier TJ, Blanchard BC, Teng YD, Synder EY, Sladek JR Jr. Cellular repair in the parkinsonian nonhuman primate brain. Rejuvenation Res. 2010 Apr-Jun;13(2-3):188-94. Review. PubMed PMID: 20370501; PubMed Central PMCID: PMC2946058.



