Short summary: Metformin has been proposed as an “anti-aging drug,” and a major clinical trial is about to get underway to test the idea. In earlier posts in the series, we’ve reviewed the animal data on metformin and outcomes relevant to intervention in aging (especially lifespan studies); human studies on metformin’s effects on a variety of health outcomes, including a flawed observational study suggesting that people with diabetes on metformin live longer than people without diabetes; and clinical trials of metformin to prevent or treat cancer or age-related cognitive decline and dementia. In this final installment, we’ll look at some of the backstory on TAME; how the trial was designed; and how it can serve as a piton to help surmount the regulatory cliffs to develop longevity therapeutics.
When it Does and Doesn’t Matter if Aging “is” a “Disease”
It’s commonplace in the prolongevist community to say that the medical conquest of aging is being held back by the fact that the US FDA and other regulators don’t recognize aging as a “disease.” To earn their shareholders a return on their investments — and therefore, to get the money that allows them to develop their therapies in the first place — biotech startups and Big Pharmas need to know that if their therapies work, they can be approved for sale. And most importantly, they need to know that regulators in rich countries like the United States, Canada, and the EU will approve them. To get their treatments to market, companies need to show regulators like FDA that their candidate therapies can prevent, treat, or cure some recognized “indication” — which generally speaking means a “disease,” and in this case, a “disease of aging.”
Accordingly, longevity biotech companies have long pitched their therapeutics to the FDA and investors alike as therapies to prevent or treat some narrowly-defined “disease” of aging that is listed as an indication on the regulators’ menu. For instance, companies have designed their trials to go after several eye diseases in which senescent cells are implicated (UNITY Biotechnology); atherosclerosis (Cyclarity Therapeutics — formerly Underdog Pharmaceuticals); age-related macular degeneration (LYSOCLEAR); age-related chronic dermatitis and idiopathic pulmonary fibrosis (IPF — the terrible age-related stiffening and scarring of the lung that slowly suffocates its victims) (Rubedo Life Sciences); and neurodegenerative aging of the Alzheimer’s type (the recent successful clinical trial of the AmyloSENS therapy lecanemab). And so on. To succeed, such trials have to show that this specific therapy targeting this specific pathway or this specific form of damage prevents the worsening of this specific disease, or that it prevents catastrophic short-term outcomes like heart attacks or amputations.
You might not have noticed that all the examples I gave above are rejuvenation biotechnologies — meaning not just longevity therapeutics in general, but the subset of longevity therapeutics that directly remove and repair specific forms of cellular and molecular damage in aging tissues. There’s a reason for that. Targeting degenerative aging using therapies that through their own properties remove and repair the damage of aging is quite unlike the route by which scientists have traditionally imagined they would develop longevity therapeutics.
From the 1960s until Dr. de Grey and colleagues’ landmark 2002 paper on the SENS strategy and still quite commonly today, scientists working in the biology of aging who hope to develop therapies targeting degenerative aging have spent their careers digging ever deeper into cellular biology. The hope is that if they dig deep enough, they will identify key nodes in biochemical pathways that either have side effects that inflict damage on the organism, or that tidy up the early stages of such damage and thus prevent it from getting fixed permanently into the functional structures of the body. In principle, scientists would then develop drugs that inhibit the former or stimulate the latter, which would (in theory) cause less damage to accumulate.
From the middle of the 20th century until 2009, this older “messing-with-metabolism” or “gerontological” approach was for some scientists merely a way to get grants in cell biology approved. And for those scientists whose motivation was genuinely to make a dent in the plague of degenerative aging, it was an exercise in pure frustration. After decades of trying one drug or nutrient after another, “gerontological” drugs and supplements had uniformly failed to move the needle on the degenerative aging process in mammals.
If you’re reading this article, you probably know that the first clear-cut success for the classical approach came in 2009, when rapamycin finally broke out from a multi-decade record of mice fed any number of presumed anti-aging drugs and supplements that had all died on their regular schedule. A seminal paper from Richard Miller and the NIA’s Interventions Testing Program was the first to show that this approach actually worked in a mammal (the laboratory mouse), and it’s continued to succeed in study after study.[2] And despite a recent report suggesting otherwise, it’s pretty clear that by any reasonable operational definition, rapamycin slows aging in mice, rats, and many other species — quite possibly including humans.
But few such examples exist: the great majority of drugs that have reached the ITP (or a massive previous effort led by Dr. Stephen Spindler) have utterly failed — and most that “succeed” have had substantial limitations. Some work only in one sex, like the SGLT-2 inhibitor canagliflozin,[3],[4],[5] the diabetes drug acarbose,[6],[7],[8],[9],[10],[11] and the non-feminizing estrogen 17-alpha estradiol [3],[5],[7],[8],[12],[13],[14]. And several others reduce the risk of dying early in life, but degenerative aging still creeps into the tissues of treated animals on its usual, merciless schedule, and they don’t live longer or stay healthier from that point onward.[15],[16]
The scientists behind TAME chose metformin despite its having failed in the ITP, and despite additional evidence — in animals, in diabetic humans, in people with or at high risk of cancer, and in people suffering with age-related cognitive decline and dementia — that it is unlikely to do much of anything for nondiabetic aging adults. But even “gerontological” drugs that do work as anti-aging agents (at least in lab animals) will likely have a hard time passing clinical trials where the endpoint is linked with some particular disease of aging. That’s because if they’re going to be effective, such agents must substantially torque metabolic pathways whose normal, regulated activity keeps us alive and healthy. The harder you push those systems outside of their evolution-driven homeostatic norm, the more likely it is that the harmful side effects of interfering with those processes will outweigh the benefits to the aging organism.
Those kinds of risks pay off when the group being treated is fairly ill with that particular disease. But the tradeoff becomes dicier when the subjects in the trial are older people who aren’t at acute risk from one specific disease of aging. To send a clear signal, such a therapy would have to simultaneously reduce the risk of many potential causes of age-related death once. That’s of course exactly what a classical anti-aging drug should do — but showing that it’s so on a budget and in a manageable time frame is a hard needle to thread.
As we’ve discussed before, this “inch deep, mile wide” property makes the need for “disease”-based outcomes a difficult hurdle for messing-with-metabolism approaches to the problem. In contrast, it’s much less of a challenge for the “damage-repair” therapies that SENS Research Foundation advocates and is working to develop. The candidate rejuvenation biotechnologies listed above that have entered or are close to human clinical trials illustrate this point. Additionally, the Accelerated Approval pathway through which the FDA approved the AmyloSENS therapy Aduhelm®/ aducanumab gave the first glimpse of how rejuvenation biotechnologies could become FDA-approved therapies, even if the approval was a flawed first step rather than a shining model for future cases. More recently, Leqembi®/lecanemab earned accelerated approval under the same regulatory pathway as Aduhelm, but without the clinical split decision or internal disagreements that made the Aduhelm decision controversial. And since lecanemab doesn’t “just” remove key beta-amyloid species from the brain, but also substantially curbs the terrible downward pull of neurodegenerative aging of the Alzheimer’s type, it’s likely to get full approval in the coming months.
The real promise of TAME has little to do with the actual potential of metformin as an anti-aging drug, and everything to do with solving this problem: getting the first piton pounded into the regulatory wall for drugs that have the kinds of broad but relatively modest effects on aging that are likely to be typical for “gerontological” drugs.
TAME will help us make the leap from the current disease-management-based approach to a future where longevity therapeutics allow us to live longer, healthier lives — not because it will prove that metformin is a wonder drug for the disease and debility of aging, but because it will set a regulatory precedent. By allowing TAME to be structured as Dr. Barzilai proposes, something that looks like “aging itself” has been established as an endpoint for clinical trials that FDA will accept as the basis to approve therapies. In effect, it will create the precedent for aging itself as an indication.

You Manage What You (Can) Measure
To prove that your therapy is slowing, arresting, or reversing aging, you need a way to measure aging. In most cases, companies seeking approval for their drugs will have to show that they reduce “hard outcomes” in clinical trials that are relevant to the indication — things like heart attacks and strokes for cardiovascular disease or progression to dialysis for kidney failure. But in cases where the biology is sufficiently well-understood, or where the alternative treatments are few and the outcomes are sufficiently dire, drugs may instead gain approval if the company can show that they improve “surrogate outcomes.”
Surrogate outcomes are measurable markers or mediators of the disease process that are linked to hard outcomes but that do not, in and of themselves, interfere with a person’s ability to live their life. For instance, a person may have dangerously high blood pressure, apoB-containing lipoproteins, or blood sugar, and yet feel more or less fine for decades. But throughout those years, their arteries (blood pressure, apoB) or eyes and kidneys (blood sugar) will have been under continuous assault by higher-than-normal cellular and molecular damage drivers. The result is that they will suffer prematurely with diseases of aging that flow from those forms of damage (heart attacks, strokes, blindness, and renal failure, respectively). So in cases where the biology is well-understood (like these), FDA has historically accepted the ability to safely lower these things as reliable surrogate outcomes to approve a drug (although they are increasingly hesitant to do so as the number of established drugs that can do this has piled up).
So if the goal is to slow, arrest, or reverse the degenerative aging process, and if we’re not going to wait 20 or even 50 years or more to complete a human lifespan study, the challenge for would-be longevity therapeutics — and especially for those that work by the older approach of tweaking metabolic pathways — is fundamentally how to measure a drug’s impact on aging in a way that FDA will recognize as success for an anti-aging therapy in a clinical trial.
Dr. Barzilai — who not coincidentally began his career working to identify the mechanism of action of metformin for diabetes — has been working to convince FDA to accept aging as an indication for the approval of therapies since the early 2000s, and this has been the core of the problem from the very beginning.[17] In the leadup to TAME, he assembled a coterie of geroscientists, medical doctors experienced in running clinical trials, and other experts to hash the question out at a repurposed Spanish castle.
There, participants weighed the pros and cons of many possible endpoints. The most robust such outcome would be death from any cause, but that would take too long if the subjects were not already suffering with overt age-related disease — and if all the subjects were already thus suffering, one specific disease (most likely atherosclerotic cardiovascular disease) would likely dominate the risk. Critics would then likely argue that the real effect was on that one disease, not on something as nebulous as aging.
So what could be used instead? The panel considered “geriatric syndromes,” a term that embraces a constellation of significant age-related disabilities, such as repeated falls, unsteady gait, incontinence, and frailty indexes. But they rejected this approach for reasons they haven’t publicly explained to my knowledge. (However, as we noted in Part 2 of this series, the ten-year follow-up of the Diabetes Prevention Program randomized trial found metformin did not lower the risk of becoming frail in the ensuing years in people with prediabetes compared to a placebo.[18] And fortunately, there’s already a smaller separate trial underway that will provide preliminary answers on this question).
Wrinkle count? Well, if anybody raised that, it was only in jest.
Epigenetic aging clocks were a very new development at the time, but if the scientists considered them as a surrogate outcome for the trial, they rejected them — which was a good call. First, to assess aging, you need to measure biological age: how much damage the biological aging process has wrought in your tissues, bringing you closer to the onset of diseases of aging and death. But at the time Dr. Barzilai’s squadron was debating the options, the only available clocks were the first-generation versions, which were built around their ability to track mere chronological age — that is, “calendar age,” or how long you’ve lived from the day you were born. Chronological age has an obvious connection to biological age, but by definition, it can’t be slowed or turned back with a drug.

The situation is better today, as researchers have designed several epigenetic age clocks designed to track biological age, not just chronological age. But even the best of these newer clocks are not yet suitable for use as goalposts for clinical trials. (We may discuss this in a future blog post, but some of the essential limitations are laid out here, and one that is critical for their use by individuals or in manageably-sized clinical trials was reported here).
Staking Out the Goalposts
Instead, the group settled on “incident multimorbidity” as the endpoint for the trial that would become TAME.[19] “Multimorbidity” means suffering from two or more of the supposedly independent “diseases of aging” at once. More than two thirds of Americans over the age of 65 have two or more chronic diseases, and half of those who have at least two have four or more.[20] And the prevalence of multimorbidity rises exponentially with age, just as individual diseases of aging do.[21]
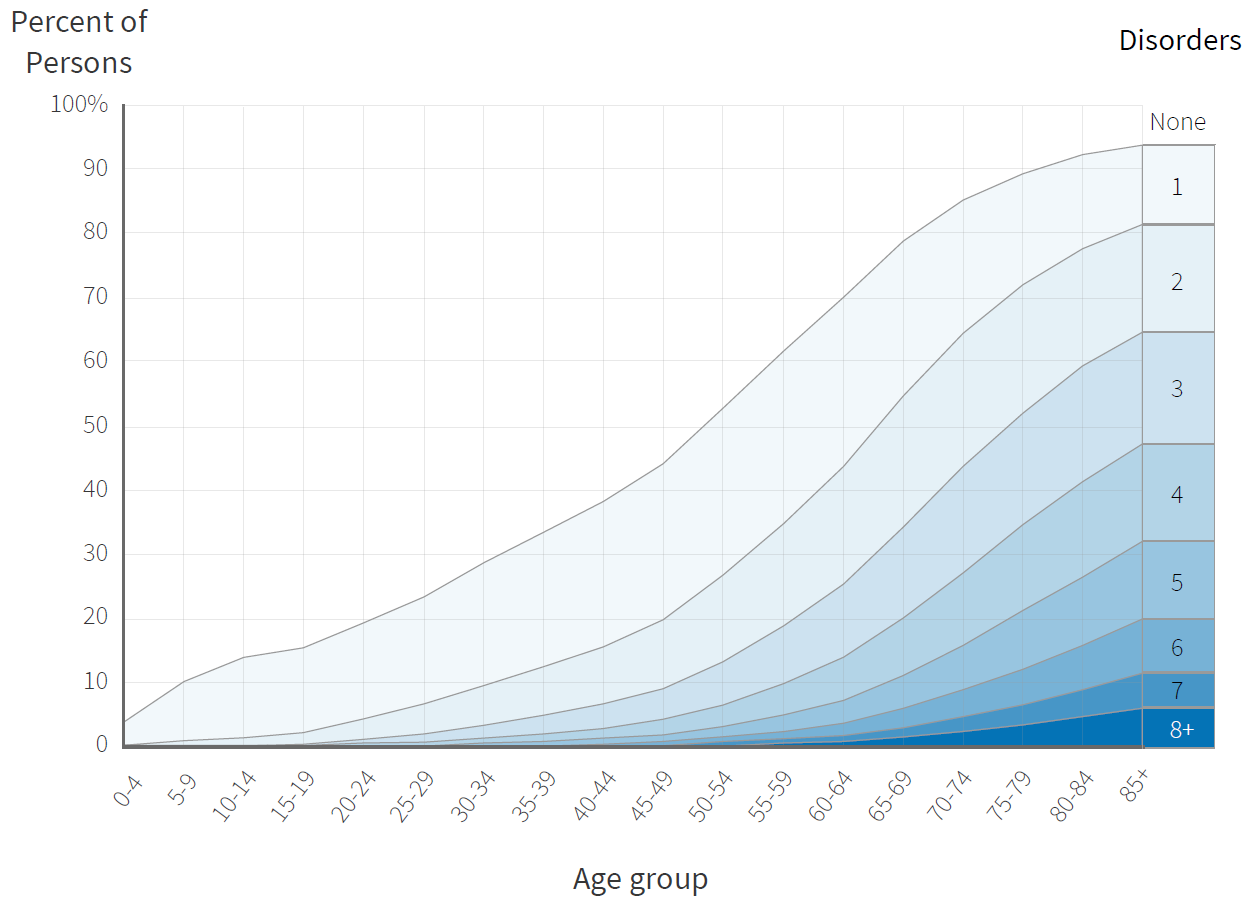
So if a single therapy were to delay the onset of any of several seemingly-independent “diseases of aging” — plus death from any cause, the risk of which is increased by multimorbidity and accelerated by aging — it would put the lie to the very idea that these diseases truly are independent. In the process, it would mark the therapy as a credible anti-aging drug.
The TAME team had considered this carefully, and had designed a simple multimorbidity composite score to serve as the trial’s endpoint. The trial would track how long it took people taking either metformin or placebo to be newly diagnosed with some particular “disease of aging” (selected from cardiovascular disease, cancer, or cognitive disease). The analysis would be based on the delay in developing each additional diagnosed disease (or death), rather than simply whether the volunteers developed one or more of them (or not) over the course of the trial.
Importantly, while the subjects in the trial will not have diabetes or a recent cancer diagnosis, they may be at elevated risk (over and above their ages) for diseases of aging based on conventional risk factors, such as being prediabetic (but not full-on diabetic); overweight (but not obese); or having high LDL cholesterol or blood pressure. And many of them will already be taking drugs to manage these conventional risk factors. So if a candidate longevity therapeutic (metformin in this case, or a future candidate in a trial modeled on the TAME template) delays the onset of these diseases, it would likely be for a reason that doctors could not cover by just managing “the usual [risk factor] suspects.” Instead, the reason for the delay would presumptively be that the drug was impacting aging.
Barzilai has argued that “There’s nothing in that clinical trial that hasn’t already been shown before with metformin”; “We are just trying to package it all up and call it aging so the FDA will approve this for aging.” As we’ve seen, none of this actually has been shown for metformin — but any genuinely effective “gerontological” longevity therapeutic ought to be able to do it in the course of a trial similar to TAME.
Even after Barzilai had received some positive initial feedback from FDA officials on TAME’s design, some of them argued that the scientists running the trial should be required to stop the study early if the oversight board saw evidence that the drug was lowering the risk of developing any one of these diseases. If so, they argued, the researchers should declare victory and send everyone home. That isolated outcome would then justify giving metformin to people at high risk of that one “disease” right away instead of waiting until the trial hit its multimorbidity outcome.
But that would be like an early Apple investor buying shares at a dollar apiece and selling when they hit five bucks instead of holding them for a bigger payoff. That approach would allow a few people with one specific age-related disease to get access to a useful therapy, but it would fail a much larger number of aging people because it would leave the real question unanswered: could this drug retard the rate of aging? Barzilai had to hold the line that multimorbidity itself would be the endpoint for the trial. And so far, he seems to have succeeded.
Now he just has to get the trial funded.
“Funding Secured”?
To actually run TAME is expected to cost $50-$75 million, with the final funding amount dictating how much data the investigators will be able to collect about the effects of the drug. And despite recent funding announcements that were met with widespread cheers in the prolongevist and professional biogerontology communities, the actual funding status of TAME seems to be in question.
Barzilai secured the first major tranche of funding for TAME from AFAR in 2019, with most of the money coming in from a donation by long-time longevity philanthropist (including Methuselah/SRF donor) Paul F. Glenn, who I note with bitter irony died less than a year later. In early 2022, Barzilai began telling people that the Saudi-backed anti-aging nonprofit Hevolution had agreed to fund the lion’s share of the rest, and in December he told Longevity.Technology that the trial was fully funded. Curiously, the accounting given in Longevity.Technology includes $35 million from AFAR, but an article published just weeks later in the Financial Times states that “So far, [Barzilai] he has $22mn, including $9mn from the US National Institutes of Health” — similar numbers to those that were cited in 2019.
And just weeks later, an unnamed representative for Hevolution appeared to deny that the organization had committed any funding for TAME, telling STAT News that it has “not yet made any decisions” about funding specific projects or ventures.
All of this is quite murky to this outside observer. Even in 2019, Barzilai had been secretive about the source of the early funding: “I can’t tell you who gave us it,” he said, “but it will be a great story one day!” The same quote, oddly, is cited regarding the $40 million commitment made in 2022 in a later Longevity.Technology piece.
Not having the money to actually launch TAME may delay the availability of longevity therapeutics in three different ways. The most obvious is that it will take Barzilai and colleagues longer to get an answer to the central question of whether metformin is an anti-aging drug. The second is that there will be a delay before longevity biotech companies will be able to point to TAME as a precedent for their own trials. It’s one thing to get the FDA to agree to a trial structure that is makes it easier for some kinds of anti-aging drugs to be tested and approved. It’s quite another to have an actual example of a well-run trial of the sort that a company is proposing to run, or to have gained experience in conducting and analyzing such trials.
The final and most way that the delayed launch of TAME could hobble the longevity biotech industry is by creating space for a bureaucratic reversal of the original FDA agreement. Convincing a panel of currently-serving FDA officials of the merits of TAME’s trial design is no guarantee that a future set of regulators will feel bound by that agreement. We’re now more than six years out from FDA’s original decision; the longer it goes without launching, the greater the odds that staff turnover, a new Administrator or senior leader, or a new Administration might seek to amend that decision or deep-six it entirely.
The TAME Toupée and the Silver(-Haired) Lining
If the money comes through, TAME could begin in the next year or two; from that point, the trial is expected to last six years. From the evidence we reviewed in the earlier posts in this series, it seems likely that the trial will end with a whimper: a few cases of diabetes will be prevented, but with no substantial effect on the multimorbidity of aging. Some metformin enthusiasts will grasp fiercely at some straw that will be plucked out of a fishing expedition conducted after the fact, but few in the prolongevist community and fewer scientists will consider it an important contender as a longevity therapeutic.

But in another sense, the trial will have succeeded from the day the first volunteer swallows a pill. The green light from FDA to run TAME the way Barzilai wanted it run will open up the express lane for many future longevity therapeutics. As he himself has stated, “Those big billionaires, they want moonshots, they want a scientific achievement that will make people say ‘wow’. TAME is not a moonshot. It’s not even about scientific achievement really, it’s more about political achievement. Metformin is a tool to get aging as an indication.”
As discussed above, FDA recognition of TAME’s pioneering trial design is less important for the class of longevity therapeutics that SENS Research Foundation advocates and is working to develop: those that directly remove and repair specific cellular and molecular lesions in aging tissues. There are more direct ways to monitor the effects of such therapies, and it will likely take no more time to get results in clinical trials than is common in current drug trials — and in some cases, less.
But TAME will open up real opportunities for developing longevity therapeutics based on the less powerful but still valuable approach of fiddling with the controls of cellular metabolism. The combination of a scientifically credible benchmark for trials using such drugs; the experience of running a large trial to show that the model is workable; and a precedent for FDA acceptance of such a trial as valid for regulatory approval will motivate scientists, startups, venture capital, and Big Pharma players to finally jump into the hitherto-lonely pool, even if metformin itself fails to pass the bar the TAME trial sets out.
The next subjects of anti-aging trials could be other repurposed existing drugs that also “mess with metabolism,” but that are supported by much better evidence than metformin is. Such candidates include rapamycin and (in males) canagliflozin and 17-alpha estradiol. In fact, Hevolution CEO Mehmoud Khan has stated that the organization will fund trials of “treatments that are patent expired or never got commercialized,” and Hevolution’s website similarly says that their “investments will be in a combination of new drug developments that have been focused on longevity from the outset, to repurposing drugs already in the market [which would include all of the above -Ed] or drugs under development that failed at pre-clinical or clinical stages on their initial indication,” so any of these drugs could already be on their priority list.
And just as a new basketball hoop acts as a magnet for neighborhood kids to come out and play, the erection of clear regulatory goalposts will trigger the launch and funding of startups working on novel anti-aging drug candidates, and the development of other as-yet-unimagined compounds and targets that will be discovered as a result of the promise that such drugs could actually one day reach aging patients.
Public Perception and Politics
TAME has garnered an enormous amount of attention from widely-read media like the Wall Street Journal, The Economist, the Washington Post, and the New York Times. It is also the focus of news articles written for technologists in Wired and MIT Technology Review, and for scientists not working in aging in Nature and Science, which are the most widely-read and reputable scientific journals on earth. This amount of sustained coverage from credible sources trumpeting the fact that serious scientists are working on anti-aging drugs is bound to increase public support — and political pressure — for investments in R&D aimed at developing longevity therapeutics.
That’s all for the good, but it hasn’t yet led to the kind of citizen action we need. For instance, the press coverage of TAME has apparently triggered a scramble of older adults trying to enroll in TAME, but many of them walk away when they realize that the investigators can’t guarantee that they’ll be given the actual drug. Many of these people instead try to convince their doctors (or a concierge medicine physician) to prescribe the drug off-label — a drug that (as we’ve seen) is likely to have negligible benefits other than for people with prediabetes. The money and energy that these people are devoting to trying to finagle a likely-useless metformin script would be better invested in participating in TAME so that we can actually get some answers, and even more so in philanthropic support of rejuvenation research, or calling their elected representatives to press for more NIH/NIA funding for the same purpose, or to reform regulations to enable more such trials.
The one great risk of TAME is the “fool me twice” problem. For years now, a wide swath of the public has heard credible scientists loudly express their optimism that metformin will for the first time bend the curve of human aging. So what happens if (as is most likely) the results of TAME are instead in line with its modest acronym? Worse: what if TAME flops entirely? Such a result could not only disappoint a lot of people, but reinforce the general public’s ingrained pessimism that anything medical can be done about the misery of aging. Having had their hopes raised and dashed by TAME could make the average person even less inclined to support research in longevity science, and make politicians even more fearful of backing it with scarce federal dollars. That could set the longevity therapeutics revolution back to a worse place than if TAME had not happened at all.
Activists Needed
No matter what the results of TAME are, the future of aging is up to us. Prolongevist activists must reach down and seize their own power to accelerate our progress in developing rejuvenation biotechnology to give us longer and healthier lives. Talk to your friends, neighbors, and coworkers about the future that longevity science promises for all of us and why you want them to share that future with you. Call and write your elected representatives to increase the NIA’s budget and to expand NIH funding to develop rejuvenation biotechnology. Invest your philanthropic dollars in supporting rejuvenation research and back crowdfunding campaigns to advance longevity therapeutics. And join the new class of activist investors who are getting longevity therapeutics startup companies off the ground and giving them the financial runway they need to move to the next level: trials testing truly revolutionary longevity therapeutics.
With your help, we at SENS Research Foundation will continue putting precious dollars to work developing longevity therapeutics that repair the damage in our tissues and will finally end the age-long aging plague. Aging will not merely be “tamed,” but vanquished, and we will live our lives free and young into an indefinite future.
Citations:
[1] de Grey ADNJ. TAME: A Genuinely Good Use of 75 Million Dollars. Rejuvenation Res. 2019 Oct;22(5):375-376. doi: 10.1089/rej.2019.2274. PMID: 31595812.
[2] Selvarani R, Mohammed S, Richardson A. Effect of rapamycin on aging and age-related diseases — past and future. Geroscience. 2021 Jun;43(3):1135-1158. doi: 10.1007/s11357-020-00274-1. Epub 2020 Oct 10. PMID: 33037985; PMCID: PMC8190242.
[3] Miller RA, Harrison DE, Allison DB, Bogue M, Debarba L, Diaz V, Fernandez E, Galecki A, Garvey WT, Jayarathne H, Kumar N, Javors MA, Ladiges WC, Macchiarini F, Nelson J, Reifsnyder P, Rosenthal NA, Sadagurski M, Salmon AB, Smith DL Jr, Snyder JM, Lombard DB, Strong R. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020 Nov 5;5(21):e140019. doi: 10.1172/jci.insight.140019. PMID: 32990681; PMCID: PMC7710304.
[4] Snyder JM, Casey KM, Galecki A, Harrison DE, Jayarathne H, Kumar N, Macchiarini F, Rosenthal N, Sadagurski M, Salmon AB, Strong R, Miller RA, Ladiges W. Canagliflozin retards age-related lesions in heart, kidney, liver, and adrenal gland in genetically heterogenous male mice. Geroscience. 2022 Aug 16. doi: 10.1007/s11357-022-00641-0. Epub ahead of print. PMID: 35974129.
[5] Snyder JM, Casey KM, Galecki A, Harrison DE, Jayarathne H, Kumar N, Macchiarini F, Rosenthal N, Sadagurski M, Salmon AB, Strong R, Miller RA, Ladiges W. Canagliflozin retards age-related lesions in heart, kidney, liver, and adrenal gland in genetically heterogenous male mice. Geroscience. 2022 Aug 16. doi: 10.1007/s11357-022-00641-0. Epub ahead of print. PMID: 35974129.
[6] Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014 Apr;13(2):273-82. doi: 10.1111/acel.12170. Epub 2013 Nov 19. PMID: 24245565; PMCID: PMC3954939.
[7] Wink L, Miller RA, Garcia GG. Rapamycin, Acarbose and 17α-estradiol share common mechanisms regulating the MAPK pathways involved in intracellular signaling and inflammation. Immun Ageing. 2022 Feb 1;19(1):8. doi: 10.1186/s12979-022-00264-1. PMID: 35105357; PMCID: PMC8805398.
[8] Harrison DE, Strong R, Alavez S, Astle CM, DiGiovanni J, Fernandez E, Flurkey K, Garratt M, Gelfond JAL, Javors MA, Levi M, Lithgow GJ, Macchiarini F, Nelson JF, Sukoff Rizzo SJ, Slaga TJ, Stearns T, Wilkinson JE, Miller RA. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell. 2019 Apr;18(2):e12898. doi: 10.1111/acel.12898. Epub 2019 Jan 27. PMID: 30688027; PMCID: PMC6413665.
[9] Garratt M, Bower B, Garcia GG, Miller RA. Sex differences in lifespan extension with acarbose and 17-α estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell. 2017 Dec;16(6):1256-1266. doi: 10.1111/acel.12656. Epub 2017 Aug 22. PMID: 28834262; PMCID: PMC5676051.
[10] Sadagurski M, Cady G, Miller RA. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell. 2017 Aug;16(4):652-660. doi: 10.1111/acel.12590. Epub 2017 May 20. PMID: 28544365; PMCID: PMC5506421.
[11] Sadagurski M, Cady G, Miller RA. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell. 2017 Aug;16(4):652-660. doi: 10.1111/acel.12590. Epub 2017 May 20. PMID: 28544365; PMCID: PMC5506421.
[12] Harrison DE, Strong R, Reifsnyder P, Kumar N, Fernandez E, Flurkey K, Javors MA, Lopez-Cruzan M, Macchiarini F, Nelson JF, Markewych A, Bitto A, Sindler AL, Cortopassi G, Kavanagh K, Leng L, Bucala R, Rosenthal N, Salmon A, Stearns TM, Bogue M, Miller RA. 17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell. 2021 May;20(5):e13328. doi: 10.1111/acel.13328. Epub 2021 Mar 31. Erratum in: Aging Cell. 2022 Jul 27;:e13672. PMID: 33788371; PMCID: PMC8135004.
[13] Garratt M, Leander D, Pifer K, Bower B, Herrera JJ, Day SM, Fiehn O, Brooks SV, Miller RA. 17-α estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell. 2019 Apr;18(2):e12920. doi: 10.1111/acel.12920. Epub 2019 Feb 10. PMID: 30740872; PMCID: PMC6413653.
[14] Garratt M, Lagerborg KA, Tsai YM, Galecki A, Jain M, Miller RA. Male lifespan extension with 17-α estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell. 2018 Aug;17(4):e12786. doi: 10.1111/acel.12786. Epub 2018 May 27. PMID: 29806096; PMCID: PMC6052402.
[15] Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008 Oct;7(5):641-50. doi: 10.1111/j.1474-9726.2008.00414.x. PMID: 18631321; PMCID: PMC2695675.
[16] Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008 Oct;7(5):641-50. doi: 10.1111/j.1474-9726.2008.00414.x. PMID: 18631321; PMCID: PMC2695675.
[17] Some of the account below draws on Hall SS. A Trial for the ages. Science. 2015 Sep 18;349(6254):1274-8. doi: 10.1126/science.349.6254.1274. PMID: 26383933; Apple S. Forget the Blood of Teens. This Pill Promises to Extend Life for a Nickel a Pop. Wired. 2017 Jul 1. Online resource. https://www.wired.com/story/this-pill-promises-to-extend-life-for-a-nickel-a-pop/ Accessed 2022-07-10; and Attia P, Barzilai N. #204 – Centenarians, metformin, and longevity: Nir Barzilai, M.D. The Drive. 2022 April 25. Online resource. https://peterattiamd.com/nirbarzilai2/ Accessed 2022-07-13.
[18] Hazuda HP, Pan Q, Florez H, Luchsinger JA, Crandall JP, Venditti EM, Golden SH, Kriska AM, Bray GA. Association of Intensive Lifestyle and Metformin Interventions With Frailty in the Diabetes Prevention Program Outcomes Study. J Gerontol A Biol Sci Med Sci. 2021 Apr 30;76(5):929-936. doi: 10.1093/gerona/glaa295. PMID: 33428709; PMCID: PMC8087265.
[19] Espeland MA, Crimmins EM, Grossardt BR, Crandall JP, Gelfond JA, Harris TB, Kritchevsky SB, Manson JE, Robinson JG, Rocca WA, Temprosa M, Thomas F, Wallace R, Barzilai N; Multimorbidity Clinical Trials Consortium. Clinical Trials Targeting Aging and Age-Related Multimorbidity. J Gerontol A Biol Sci Med Sci. 2017 Mar 1;72(3):355-361. doi: 10.1093/gerona/glw220. PMID: 28364543; PMCID: PMC5777384.
[20] Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013 Apr 25;10:E61. doi: 10.5888/pcd10.120137. PMID: 23618541; PMCID: PMC3652723.
[21] Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012 Jul 7;380(9836):37-43. doi: 10.1016/S0140-6736(12)60240-2. Epub 2012 May 10. PMID: 22579043.
See also the other posts in this series:
- Part 1: on the animal data on metformin and aging;
- Part 2: on some human studies on metformin, including a flawed observational study that created the illusion that diabetics on metformin actually live longer than people without diabetes;
- Part 3: on human trials of metformin to prevent or treat cancer;
- Part 4: on human studies on metformin and age-related cognitive decline and dementia; and
- Addendum: More Studies on Metformin and Survival
- Addendum: Monkeying With the Clocks Via Metformin