
Short summary: Metformin has been proposed as an “anti-aging drug,” and a major clinical trial is about to get underway to test the idea. There’s not much chance that metformin will turn out to slow the rate of aging in humans, but TAME may help pave the way to important future trials of longevity therapeutics. In Part One of this four-part series, we’ll look at the animal studies that got many scientists excited about metformin in the first place and see where they went wrong.
Knowledgeable sources have reportedly told the press that the well-funded longevity therapeutics nonprofit Hevolution Foundation has tentatively agreed to provide the last tranche of funding for the long-delayed Targeting Aging with Metformin (TAME) trial. TAME is a large-scale human trial to see if the cheap, safe diabetes drug metformin can slow aging in a cohort of several thousand aging but nondiabetic people across the United States. Update, October 2022: a spokesperson for Hevolution has recently cast that claim into doubt, telling the health science news site STAT that it has “not yet made any decisions” about funding any particular project or venture.
While undeniably meriting at least one out of three cheers, the implications of this decision for the future of longevity therapeutics are decidedly mixed. On the one hand, we can say with a high level of confidence that metformin is a poor candidate as an anti-aging therapy. But on the other, the trial itself may pave the way for better candidate longevity therapeutics in the future, if the prolongevist community can navigate the craters its likely crash landing will create.
Rush to Judgement
The notion that metformin might have anti-aging effects began with a series of studies in mice and rats who weren’t dying as a result of the degenerative aging process, but from mutations that made them prone to cancer.[1],[2] But the first study of metformin’s effects on otherwise-healthy aging rats found no effect on median or maximum lifespan.[3]
There were some reasons to be skeptical of the negative result, and hopes were raised by another study published around the same,[4] which time found that metformin was beneficial in a stock of mice that, although short-lived for the species, are certainly much better test animals than grossly abnormal, mutant mice. But the significance of that study was undermined by a followup study from the same scientific team, which tested metformin in adult and older mice.[5] In their first, positive-looking metformin study, the scientists began treating the mice with metformin immediately after weaning. (To think about this, imagine your reaction if your doctor advised you that to avoid the worst impacts of your diagnosis (aging), you should have started taking a potential longevity drug as a toddler and then kept it up throughout your life). When, in the follow-up study, the scientists gave the same dose of metformin to the same stock of mice, but only started doing so once the animals were either young or middle-aged adults.
When mice began treatment at nine months of age (the human equivalent of one’s early 30s), the scientists found that metformin had only a small effect on median survival — and no effect on maximum lifespan. And when they held off dosing the mice until they were 15 months old (human-equivalent age 50), the drug had no effect at all.[5] Plus, in this stock of mice, metformin quite substantially reduced the animals’ weight — which might well be the explanation for the slight increase in median survival in the young adult mice, and which doesn’t happen so dramatically in most mouse strains or in nearly any humans.
The animal study most often cited as evidence that metformin slows aging in lab mice[6]is no such evidence at all. The investigators tested two doses of metformin in healthy, wild-type, nonobese mice. At the lower of the two tested doses, metformin increased the animals’ mean survival by a paltry 4-6%, and had no effect on maximum lifespan, meaning that the drug prevented a small number of deaths during and before middle age, but had no effect on aging. And when the mice were given the higher dose of metformin, it actually shortened the animals’ lives![6]
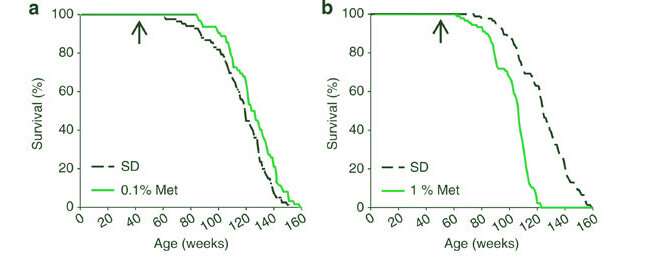
Moreover, even the “low” dose of metformin was already so high that the levels of drug in the animals’ serum and liver were “considerably higher than seen in the serum of diabetic patients treated with metformin [my emphasis]”.[6] So how would one realistically attempt to translate this into human use? Based on the levels of metformin to which the animals’ tissues were actually exposed, you’d need a much higher dose than is used by diabetic patients to get even a very small change in median life expectancy. But yet “high-dose” metformin shortens life. So how high is too high?
A recent study made an attempt to suss out that question, and came up short.[7] The researchers tested two doses of metformin in rats in late life — a time in current lifespans that is more analogous to the ages of the people who will be enrolled in TAME, and at which most people will be the most motivated to seek out a longevity therapeutic. One dose was the same as the lower dose that had increased mean life expectancy in the two-dose study; the other was higher than that, but substantially lower than the much higher dose that proved to be toxic in the earlier study. Neither dose had any effect on median or maximum lifespan.[7]
Failing the Gold Standard
The best animal study to test metformin as a potential anti-aging drug was conducted as part of the National Institute on Aging (NIA)’s Interventions Testing Program: a rigorous, systematic effort to test conventional “messing with megabolism” anti-aging agents. ITP studies are designed with several features that make them a better test than the great majority of studies of whether a potential longevity therapeutic actually works (in mice!).
First, each time the ITP tests a potential longevity therapeutic, the lifespan study is done not just once, but three times independently in parallel, with three separate cohorts of mice living out their lives at three independent research sites, cared for by three different groups of scientists. So a candidate longevity therapeutic that succeeds in the ITP program will have extended lifespan not in just one isolated lab, but by three labs independently, essentially eliminating the chance of a pure statistical fluke or a result that can’t be replicated. Second, ITP tests all candidate longevity therapeutics in a healthy, genetically-diverse mouse population, which better resembles the normal human population than the genetically homogenous mouse strains that have been the workmice of biomedical research, which may have genetic quirks that make successful results meaningless for most or all humans. And third, all three groups of scientists that run the ITP studies in parallel with each other have a lot of experience in running well-conducted lifespan studies, which greatly reduces the risk of garbage-in-garbage-out results like (say) the recent nothingburger with glycine and n-acetylcysteine.[8]
And when the ITP researchers put metformin to the test, the result was unambiguous.[9] It did not extend the lives of the mice at any site. It did not even cause the modest reduction in early deaths seen in the previous, widely-cited study.[6] Metformin simply has no effect at all on lifespan in normal, healthy mice.
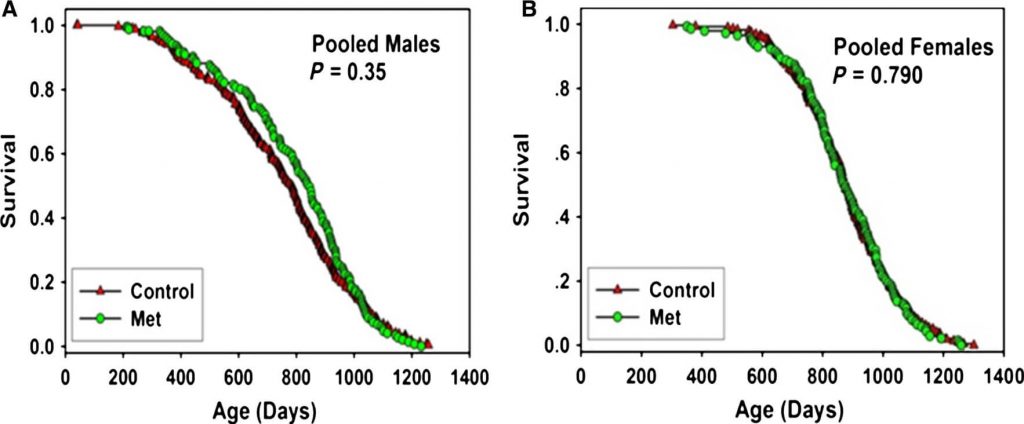
This strongly suggests that previous reports of life-extending effects of metformin were either due to random chance, or were because metformin had an effect on some metabolic quirk peculiar to the mouse strain in the study, or were the result of poor experimental design or the researchers’ lack of understanding of what it takes to keep mice alive and thriving for the course of their adult lives up to the usual limits of their lifespans.
Bad Medicine
Meanwhile, several studies that have reported that metformin is actually harmful to otherwise-healthy aging mice have never gotten the attention of the one overemphasized positive-looking paper — a paper that (remember) itself reported metformin to be toxic at the higher of two tested doses.[6] For example, one study found that when metformin is given to mice in late middle age, it worsens age-related heart degeneration and shortens the mice’s lives (although this colony of mice was already short-lived, despite having normal genetics).[10] The drug also turned down the expression of genes involved the production of cellular energy, which was reflected in the reduced availability of cellular energy in the mice’s hearts.
Meanwhile, metformin turned up the expression of genes involved with production of structural proteins (extracellular matrix (ECM)), which sounds good — except that excessive, disordered ECM (fibrosis) is one of the things that stiffens the aging heart, making it a less effective pump. And indeed, the metformin-treated mice in the study had worse fibrosis than animals not given the drug.[10] (For the record, however, another study — this one conducted in older rats instead of middle-aged mice — found that metformin had a beneficial effect on the heart, and while it still didn’t lengthen the animals’ lifespan, it at least didn’t shorten it).[7]
And don’t get me started on the sorry state of the scientific literature on metformin’s effects in worms, flies, and cells in a dish. Such studies form the basis for most of the claimed mechanisms by which some scientists argue that metformin should slow aging, such as activation of the low-energy sensor AMPK or suppressing the activity of Complex I of the mitochondrial electron transport chain. I won’t spend much time on these: if you’re really interested, have a look at an open-access review that digs deep into the microscopic weeds.[11] I will say in that the data are such a scattershot mess; that the doses used in many of these studies are laughingly unrealistic; and above all that looking at what a drug does to isolated cells or to organisms that don’t have arteries, develop cancer, or have skeletal systems is an even less reliable way to predict what it might do in humans than are studies in lab rodents. And even drugs that work in our fellow mammals have a greater than 90% fail rate when tested in the species of interest: we humans.
Citations:
[1] Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, Re F, Franceschi C. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005 Aug-Sep;40(8-9):685-93. doi: 10.1016/j.exger.2005.07.007. PMID: 16125352.
[2] Anisimov VN, Egormin PA, Bershtein LM, Zabezhinskii MA, Piskunova TS, Popovich IG, Semenchenko AV. Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med. 2005 Jun;139(6):721-3. doi: 10.1007/s10517-005-0389-9. PMID: 16224592.
[3] Smith DL Jr, Elam CF Jr, Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB. Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci. 2010 May;65(5):468-74. doi: 10.1093/gerona/glq033. Epub 2010 Mar 19. PubMed PMID: 20304770; PubMed Central PMCID: PMC2854888.
[4] Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008 Sep 1;7(17):2769-73. doi: 10.4161/cc.7.17.6625. Epub 2008 Sep 11. PMID: 18728386.
[5] Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, Poroshina TE. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY). 2011 Feb;3(2):148-57. doi: 10.18632/aging.100273. PMID: 21386129; PMCID: PMC3082009.
[6] Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. PMID: 23900241; PMCID: PMC3736576.
[7] Herbst A, Hoang A, Kim C, Aiken JM, McKenzie D, Goldwater DS, Wanagat J. Metformin Treatment in Old Rats and Effects on Mitochondrial Integrity. Rejuvenation Res. 2021 Dec;24(6):434-440. doi: 10.1089/rej.2021.0052. PMID: 34779265; PMCID: PMC8742278.
[8] Kumar P, Osahon OW, Sekhar RV. GlyNAC (Glycine and N-Acetylcysteine) Supplementation in Mice Increases Length of Life by Correcting Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Abnormalities in Mitophagy and Nutrient Sensing, and Genomic Damage. Nutrients. 2022 Mar 7;14(5):1114. doi: 10.3390/nu14051114. PMID: 35268089; PMCID: PMC8912885.
[9] Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Martinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016 Oct;15(5):872-84. doi: 10.1111/acel.12496. Epub 2016 Jun 16. PMID: 27312235; PMCID: PMC5013015.
[10] Zhu X, Shen W, Liu Z, Sheng S, Xiong W, He R, Zhang X, Ma L, Ju Z. Effect of Metformin on Cardiac Metabolism and Longevity in Aged Female Mice. Front Cell Dev Biol. 2021 Jan 26;8:626011. doi: 10.3389/fcell.2020.626011. PMID: 33585467; PMCID: PMC7877555.
[11] Mohammed I, Hollenberg MD, Ding H, Triggle CR. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front Endocrinol (Lausanne). 2021 Aug 5;12:718942. doi: 10.3389/fendo.2021.718942. PMID: 34421827; PMCID: PMC8374068.
So what do the human studies tell us? That’s the question we’ll dig into in Part Two of this series!
See also the other posts in this series:
- Part 2: on some human studies on metformin, including a flawed observational study that created the illusion that diabetics on metformin actually live longer than people without diabetes;
- Part 3: on human trials of metformin to prevent or treat cancer;
- Part 4: on human studies on metformin and age-related cognitive decline and dementia; and
- Part 5: on the backstory on TAME and how it might impact the push for longevity therapeutics.
- Addendum: More Studies on Metformin and Survival
- Addendum: Monkeying With the Clocks Via Metformin



