
SENSible Question: One of the reasons scientists test longevity therapeutics in mice first instead of people is that lifespan studies would be impractically long in humans, whereas they can be done in 3-4 years in mice. Wouldn’t it actually accelerate progress much faster if we instead did most testing in far shorter-lived animals, like the roundworm C. elegans or the fruit fly Drosophila?

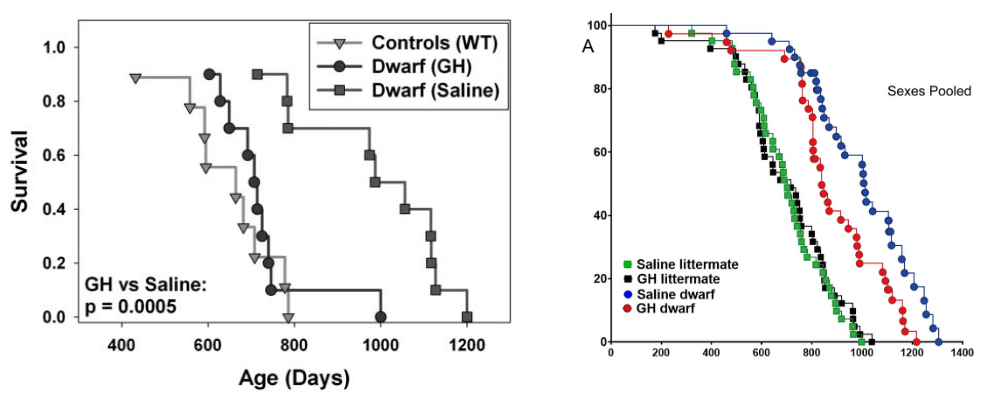
Additionally, some interventions that work in C. elegans act by altering the worms’ early developmental processes, which isn’t terribly helpful to those of us who (as founding CSO de Grey is fond of saying) “have the misfortune of already being alive.” That’s also becoming increasingly evident in mice. We’ve known for about twenty years now that mutations that block IGF-1 signaling in mice slow down their aging and extend lifespan. But those mutations dampen down signaling through these pathways throughout the animals’ entire lives, from the time they’re in the womb until the day they die. To take advantage of that discovery and develop a longevity therapeutic that would work in middle-aged and older adults, a large part of the anti-aging effect would have to be due to the hormone still being low during adulthood.
Instead, studies have shown that almost all the benefit of the “Ames mutation” goes away if growth hormone production is brought back to normal during the very earliest period of life — before the mouse has even stopped suckling its mother, let alone developed into a mature adult. After that, it’s too late to benefit from low growth hormone levels: female Ames mice live no longer than normal mice, and male mice only a little longer, despite being in in a low-growth hormone state throughout adolescence and adulthood.
Crawling Our Way Forward?
But all of the preceding examples apply to studies based on trying to usurp the regulation of metabolism to slow the aging process down. SENS Research Foundation is instead grounded in the direct “damage-repair” strategy of SENS. If we’re going to use an organism as a test animal for rejuvenation biotechnology, it has to accumulate similar kinds of aging damage as we do, and it must do so in similar tissues and with similar pathological results. And here C. elegans and Drosophila just aren’t qualified for the job.
For instance, C. elegans don’t live long enough to accumulate cells overtaken by mitochondrial DNA deletions, and there is no clear link between other kinds of mitochondrial DNA damage and the rate of aging in these worms. It is not clear to this author whether nuclear DNA accumulations accumulating in the cells of aging C. elegans contribute to their aging either — but if they do, it’s not through causing cancer, since the worms don’t develop this disease because their mature bodies are composed entirely of cells that don’t divide. Granted that the medium-term “damage-repair” strategy for nuclear mutations is first and foremost to build an impregnable wall against cancer, the fact that they don’t develop cancer again makes them a lousy model for testing SENS therapies (especially when shooting for the kind of multi-component approach that’s needed to reach Robust Mouse Rejuvenation (RMR)).
The lack of dividing cells is also why C. elegans aren’t susceptible to the many diseases that afflict epithelial tissues like the skin or the linings of many of our organs. That includes atherosclerotic cardiovascular disease (ASCVD), the disease of the arteries that is the number one single-disease cause of death worldwide. In fact, C. elegans are so small that they don’t even have hearts or circulatory systems as we would recognize them in mammals. Instead, the worms rely on diffusion and the rhythmic pumping of their feeding tube to spread oxygen, waste gas, and fuels around their tiny bodies. And the major arteries are possibly one of the first places in the body where rejuvenation biotechnology will target AGE crosslinking, another target for SENS. So already, that’s the two greatest causes of death in humans that are off the table if C. elegans are the test animal of choice.
C. elegans also have no bones, so no osteoarthritis or osteoporosis either. And they lack any of the cells dedicated to the immune system. Instead, one of their primary defenses against pathogens is called “behavioral avoidance” — meaning that they literally run (all right: wriggle) away from dangerous microbes! So the worm model is also blind to the catastrophic failure of the immune system with age, and we can’t meaningfully use them to test damage-repair strategies to remedy it.
With all those causes of death passing by C. elegans, what exactly ends their incredibly short lives? Mostly, it appears to be pathologies of their feeding tube.
Fly Away Home?
Although significantly closer to mammals than roundworms are, the fruit fly Drosophila also ages very differently from mammals like us at the structural level. For instance, Drosophila don’t lose dopaminergic neurons in their brains as they age. These specialized neurons produce the signaling molecule dopamine, and their loss with age is responsible for the more prominent symptoms of Parkinson’s disease. Their brains also don’t naturally suffer the age-related accumulation of the most important intracellular and extracellular aggregates that drive this and other neurodegenerative aging diseases, unless scientists genetically modify them with mutated versions of these human proteins.
(It’s often said that mice and rats don’t either — but this isn’t quite true. Completely normal Fisher 344 x Brown Norway rats accumulate soluble beta-amyloid protein as they age, albeit not plaque, and so do mice subjected to experimental high blood pressure. And normal Wistar-Kyoto rats develop neurofibrillary tangles, though the tangles are restricted to the branching arms of the neuron instead of extending into the cell’s main body. Plus, both mice and rats accumulate lipofuscin in their brains as they age — lipofuscin that is chemically very similar to the junk in human brains. Instead, shortly before a Drosophila dies, a mysterious sudden spike of an unknown fluorescent compound occurs in their eyes and elsewhere in their bodies (but not specifically in their brains), leaving a scene straight out of a 1950s zombie movie.

Drosophila have been useful for studying the genetic and biochemical basis of excess cell proliferation and the role of specific genes in cancer biology, but some of the differences are stark. Some of the most important genes that keep our cells from becoming cancerous (such as p53, Rb, and APC) don’t even cause cellular overgrowth in the fruit fly, let alone full-on cancer.
And while Drosophila do develop abnormal growths in different tissues, many of these disorders are congenital genetic disorders rather than the result of mutations acquired because of aging. These tumors are not true cancers, and often behave very differently from cancer as it occurs in humans. And the one disorder of excessive cell growth and loss of differentiation that is a prevalent cause of death in aging flies — perhaps the most common — is “the exception that proves the rule:” a growth of abnormal cells kills flies through loss of barrier function in their guts, which is not something we would recognize as cancer.
Whereas C. elegans don’t suffer cancer, and Drosophila tumors only “rhyme” with it, cancer is the number one cause of death in lab mice — and even when they die of cancer, aging mice die with many of the other pathologies of the aging body that plague humans, and accumulate lesions from of all categories of aging damage targeted by SENS.
That said, aging fruit flies’ bodies do share more specific aging pathology with humans than roundworms do. One important example is that — like the human heart — Drosophila heart structure and function also degenerates with age. Their heart muscle cells become increasingly disorganized, they become increasingly susceptible to erratic heartbeats (arrhythmias), and their hearts stiffen due to abnormal extracellular matrix deposition, leading to heart dysfunction that in some ways resembles human heart failure. Their hearts also lose cells as adults — but because of ongoing programmed cell death rather than heart attacks or other insults as happens in humans.
Yet while certainly not good for aging flies, it’s not clear that any of these heart lesions actually cripple or kill them as they so often do in humans. Certainly they don’t get atherosclerosis or hypertension, which are the drivers of cardiovascular death in humans. Indeed, while flies accumulate AGE of a sort, the known AGE species in Drosophila are the molecular equivalent of barnacles rather than the AGE that is the principal target for SENS rejuvenation biotechnology in the major arteries.
By contrast, mice develop human-like heart dysfunction, fibrosis, arterial stiffening, and dysfunction of the blood vessels with age. And although they don’t develop atherosclerosis when fed a standard diet, non-mutant mice do develop atherosclerotic lesions if fed a diet overloaded with saturated fat, cholesterol, and “supplemental” bile acids.
Testing longevity therapeutics in Drosophila is also made tricky; complicated by the large differences in lifespan that can result from dietary minutia that would make little difference in the life of a mouse or (wo)man, and by the need to keep the animals free from a parasitic bacterium called Wolbachia that kills most fly strains prematurely. But equally, protocols intended to keep the animals free of this parasite can also shorten the lives of some fly strains. An additional fly in the ointment (the reader will look mercifully on the pun) is that scientists usually deal with Wolbachia by regularly treating the fly colony with antibiotics — but doing so inevitably confounds experiments with the confusing inconsistency of reports of the gut microbiome in fly aging and lifespan, and its likely role in influencing aging and its diseases in humans.
For goodness’ sake, you can substantially extend the lifespan of Drosophila by putting the little pests in the fridge! Even the most enthusiastic fan of Wim Hof can’t expect it to work that way in mammals.
Beyond Biology: Other Reasons to Stick with Mice
We’ve seen above that as a matter of sheer biology, short-lived invertebrates like C. elegans and Drosophila are not good choices to test rejuvenation biotechnologies. But there are other reasons to prefer mice that go beyond such narrowly scientific ones.
For one thing, most people find lab mice to be sympathetic, cute creatures, and their youthful or degenerative aging are obvious to the naked eye. These features make them better animal ambassadors for the effects of rejuvenation biotechnology than creepy-crawlies of one kind or another. Those same characteristics are even more true of our pets, which is one reason why Dr. Matthew Kaeberlein is running “trials” of rapamycin in pet dogs, even though they live even longer than mice do and thus take yet more time to show results. Since part of the goal of RMR is to seize the imagination of the public, this “cuteness factor” gives mice a decided leg up over animals that either don’t have legs at all, or whose legs are supported by an articulated exoskeleton.
Additionally, FDA and its counterparts in other wealthy countries insist that developers test their would-be therapies in mammals, one of which must be a rodent. If the goal is to get our therapies into humans quickly, we save time by cutting out the middle-worm and going directly to mice, which will in turn bring us closer to human trials.
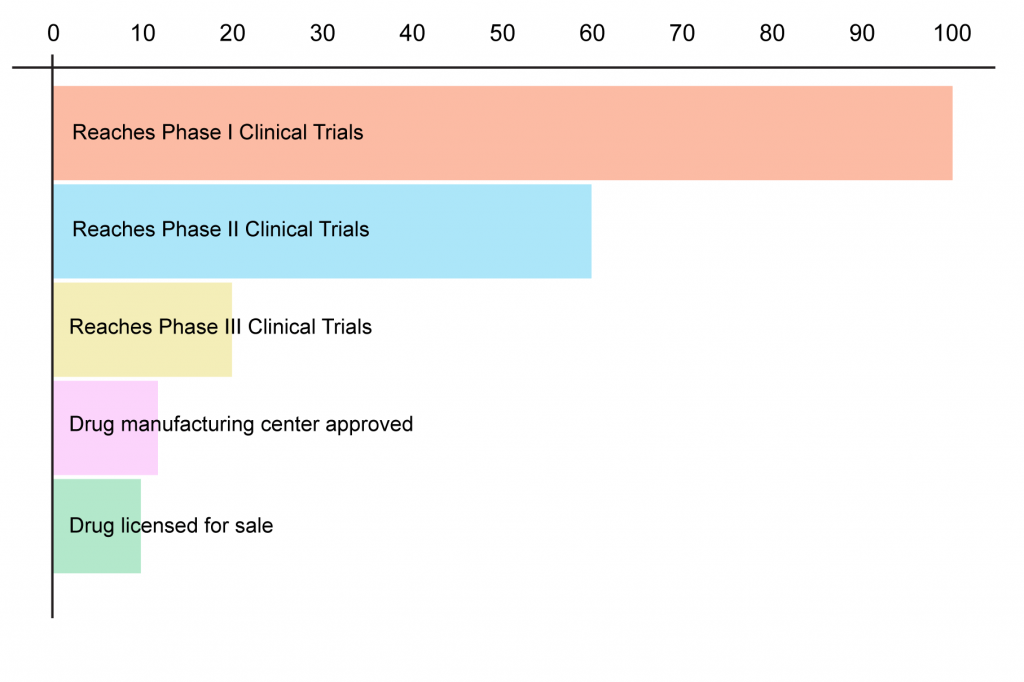
And finally, it’s important to remember that even after completing the mouse and other animal studies required to start human testing, far too many drugs fail to prove themselves safe or effective in humans — many of them at the very first step along the way.
Because they directly target stable cellular and molecular damage rather than trying to reach them through the tangled pathways of metabolism, “damage-repair” strategies are in principle less prone to foundering on the shoals of the metabolic quirks of some test animal than are conventional drugs. No model is perfect, but we should make the best use of the animal partners we have. All the medicines that have saved human lives today — from antibiotics, to clotbusters, to statins, to recombinant blood proteins for haemophiliacs — went through this path. We can now take it to greater heights by conquering aging, the plague that has been the ultimate target of medicine since the dawn of science.




