A comprehensive suite of rejuvenation biotechnologies must include the removal of extracellular aggregates from aging cells and tissues. The most clinically-advanced such biotechnology is immunotherapy against aggregated beta-amyloid protein (Aβ), a characteristic neuropathological lesion that accumulates in the brain in Alzheimer’s disease (AD) patients and as part of “normal” brain aging.(1)
The promise of active and passive Aβ-targeting vaccines is high, but experimental and clinical testing of such therapies have revealed some of their limitations. Immunotherapeutics currently in clinical development rely in different ways on the mobilization of the patient’s immune processes. Active Aβ immunotherapy (such as AN1792) involves aggregates of Aβ itself (along with adjuvants), mobilizing an active Aβ-targeting immunoglobulin G (IgG) antibody response. Therapeutic efficacy thus depends on the patient’s immune response to vaccination, which notoriously declines with aging; indeed, in the phase I trial of AN1792, serum anti-Aβ antibody titers were low, exceeding the secondarily-defined threshold of 1:1,000 in less than a quarter of AD patients. This low response was enhanced in the later Phase IIa trial by reformulation of the adjuvant, which increased the immunological response rate to nearly 60%, but generated a shift from a predominantly Th2 response to an inflammatory Th1 T-cell response. Unfortunately, this aggressive T-cell response led to the emergence of meningoencephalitis in ~6% of patients, leading to the early cessation of the trial.(1) Second-generation active vaccines are in development that are designed to minimize these risks, but may to varying degrees necessitate a difficult balance of pro- and noninflammatory mechanisms. Moreover, should these or other side effects occur as a result of active vaccination, host immune response will persist for some time until anti-Aβ antibody titers decay, potentially requiring the extended administration of immunosuppressive therapy to avert major adverse events.
Passive Aβ-targeting vaccines (bapineuzumab is furthest down the clinical pipeline) administer such IgGs generated ex vivo directly to patients, eliminating the risks associated with activation of host active immune response, and allowing for immediate cessation of therapy should adverse events occur. But in clinical trials, passive vaccination with bapineuzumab has exhibited side-effects of its own, causing vascular microhaemorrhages in a minority of apolipoprotein E ε4 carriers, thought to be caused by IgG-bound Aβ monomers or small oligomers being deposited in the cerebral vasculature following mobilization from stable plaques;(1) such effects might be exacerbated by the relative stability of the IgG-Aβ immune complexes as they bind to BBB receptors, and the activation of inflammatory mediators.(4) Parallel effects have been observed following passive vaccination in transgenic mouse models of AD.
Moreover, monoclonal Aβ-targeting antibodies are by their nature quite expensive, and are likely to require large (stoichiometric) quantities of IgG antibodies to be administered on a regular and frequent schedule to maintain therapeutic levels of the antibodies in the patient. These features might make the cost of ongoing therapy prohibitive, especially for an extended schedule of preventive therapy in the large number of”normally”-aging individuals with significant Aβ deposition but without existing cognitive impairment who are in principle the best candidates for such therapy.(3) Anticipated frequencies of dose administration could also be problematic: an early Phase I trial of bapineuzumab, for instance, estimated on the basis of pharmacokinetic data that a 13-week dosing interval would be necessary to secure ongoing therapeutic benefit.(4) In addition to requiring large financial outlays for both the immunotherapy agent itself and for the health professionals who would administer them, frequent dosing would significantly inconvenience to patients, likely leading to noncompliance in asymptomatic patients.
An ideal Aβ immunotherapy would thus not depend on the patient’s immune system for effectiveness or safety, but would have an “intrinsic” mechanism of action, and would have a high ratio of aggregate clearance to antibody dose counts and frequency of administration.
Dr. Sudhir Paul and his group at the Chemical Immunology Research Center at the University of Texas-Houston Medical School have made significant progress with a promising novel approach to Aβ immunotherapy that promises to deliver on all of these fronts. They have identified, purified, and characterized endogenous catalytic antibodies that with direct hydrolytic activity against these pathological aggregates, and have isolated rare chain fragments that are even more potent; they are advancing their research progressively toward developing these antibody fragments into a therapy for AD and brain aging.
Catalytic Immunotherapy Against Beta-Amyloid
As Dr. Paul’s group report,
Michael Sierks at the University of Arizona observed that two recombinant antibody light-chain subunits with [vasoactive intestinal peptide]-hydrolyzing activity also displayed cross-reactive Aβ-hydrolyzing activity. This prompted us to screen monoclonal and polyclonal preparations of intact antibodies for this activity. … Naturally occurring immunoglobulinM (IgM) class antibodies that hydrolyze Aβ and inhibit Aβ aggregation were identified. … Most IgM preparations from nondemented elderly humans displayed detectableAβ40 hydrolytic activity. … The production of these antibodies increases as a function of age, ostensibly reflecting an attempt by the immune system to protect against the deleterious effect of Aβ accumulation in old age. [Similarly, “Twenty two of the 25 IgM preparations from undemented elderly humans studied displayed detectable (125)I-Aβ40 hydrolytic activity varying over a 118-fold range. [Fig. 1(A) below]… IgMs from [an age-matched] AD group displayed superior hydrolytic activity [Fig. 1(B) below]”(5)] …
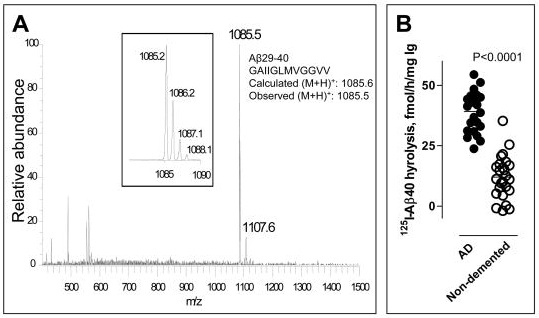
Figure 1: Variation in Aβ-catalytic activity of IgM isolated from nondemented elderly (1(A)) and in that from AD patients (n=23, Fig 1(B), left). vs. elderly, nondemented controls (n=25, right). (From (4))
As previously discussed, purified, pooled human immunoglobulins for intravenous delivery (IVIG) have already shown evidence of efficacy as an anti-Aβ immunotherapy. It would be reasonable to assume that IVIG would include Aβ-hydrolyzing IgM clones. However, Paul’s group has found no evidence of such activity:
IgG purified from the plasma of old humans by an affinity fractionation procedure involving acid treatment also hydrolyzed Aβ detectably, albeit at levels smaller than the IgM fractions from the same humans. [Yet] No Aβ hydrolysis was detected by [either of 2 approved] IVIG preparations … [Nonspecific small model] peptides were also cleaved poorly by IVIG preparations compared with IgG purified by the acid-affinity purification procedure. Evidently, the IgG catalytic activity does not survive the purification procedures used to prepare IVIG. Recent studies on a recombinant catalytic antibody fragment also suggest the sensitivity of the catalytic site to conformational perturbations …(5)
We searched for Aβ -hydrolyzing recombinant human immunoglobulin variable (IgV) domains in a library composed of ~10[to the power of]7 clones from humans with systemic lupus erythematosus, an autoimmune disease associated with enhanced production of catalytic antibodies … A minority of clones possess unusual IgV structures generated by DNA manipulation errors that inevitably accompany repeated nucleic acid replication and cloning cycles over the course of library construction. By random screening and covalent phage-IgV selection using an Aβ40 analog … we isolated rare IgV clones capable of hydrolyzing Aβ40 rapidly. The rate of Aβ hydrolysis by the IgVs was 3-4 orders of magnitude greater than the naturally occurring IgMs … For example, from its turnover number determined at excess Aβ concentration, a single [candidate catalytic antibody fragment] molecule will degrade 4320 Aβ molecules in 3 days. The rate of catalysis is comparable to that of neprilysin, an enzyme that has received attention as a potential Aβ-clearing drug. In unpublished studies, we observed that minimizing conformational perturbations of the IgVs during purification improves the catalysis rate by another order of magnitude….(2)
The IgVs are also unique by virtue of their specificity for Aβ. … The IgVs did not hydrolyze irrelevant proteins and model protease substrates customarily employed to monitor catalyst specificity … suggesting the feasibility of specific Aβ clearance with little or no damage to other proteins. … Neprilysin and other Aβ-hydrolyzing enzymes, in contrast, hydrolyze irrelevant polypeptides. (2)
The fact that these IgV clones hydrolyze, rather than sequester, Aβ offers numerous theoretical advantages over immunotherapies currently in clinical trials. First, it could dramatically reduce the amount of antibody required for Aβ clearance relative to passive immunization, because a small number of high-activity catalytic enzyme fragments could hydrolyze a large number of Aβ molecules, likely reducing the cost of each round of therapy. Secondly, because the enzyme has autonomous hydrolytic activity, rather than relying on other immunologic response, it should not be limited by the immune status of recipients, nor cause inflammatory or other immunologic side-effects. Moreover, the effectiveness of a catalytic IgV immunotherapy should powe much less risk of vasogenic edema as a consequence of sequestration and efflux of IgG-bound Aβ: the aggregates would be permanently hydrolyzed, rather than sequestered into stable immune complexes and then transported through the cerebral vasculature and out through the BBB, and then left to circulate systemically before ultimate nonspecific degradation in the circulation or disposal by excretion.(2,5) (Figure 2) And, finally, the high specificity of the IgV clones for Aβ further implies an absence of off-target adverse effects.
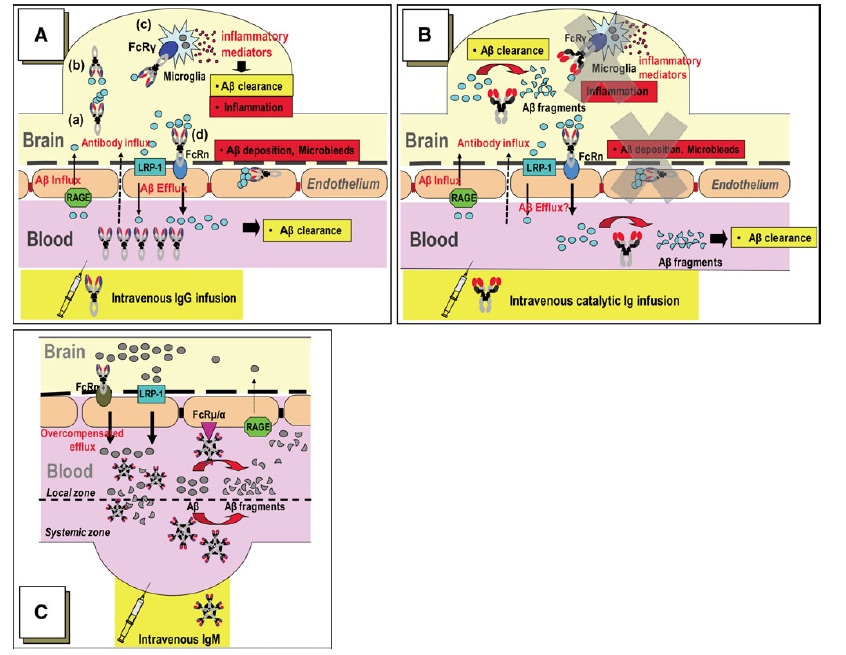
Figure 2. Comparison of mechanisms of BBB Aβ efflux by peripheral immunotherapies. (A): Aβ sequestered by IgG are taken up by microglial Fcγ receptors for digestion, and IgG-bound Aβ may be taken up by neonatal Fc receptors (FcRn) at the BBB, leading to BBB transcytosis and peripheral efflux. The former is likely responsible for the inflammation associated with active vaccination with AN1792; the latter appears to contribute to vasogenic edema following deposition of stable complexes in the cerebral vasculature. (B): catalytic IgM or IgM-derived IgV clones should abrogate these potentially deleterious effects. Instead, (C) on the basis of the observed increase in peripheral Aβ in Alzheimer’s model mice administered human Aβ-targeting IgM, Dr. Paul’s group hypothesizes that as Aβ hydrolysis in the periphery increases the trans-BBB Aβ concentration gradient, IgM binds to BBB Fc receptors for IgM (Fcμ/α) and promotes direct Aβ transcytosis by FcRn. Figure from (6)
“Catalytic IgM preparations from healthy humans inhibited Aβ aggregation, dissolved preformed Aβ aggregates, and inhibited the toxic effect of Aβ oligomers on cultured neuronal cells.”(5) And there is preliminary evidence of efficacy in a mouse model of AD, based on isolated human Aβ-cleaving IgM rather than the more therapeutically optimized IgV clones:
In a preliminary study, a preparation of catalytic human IgM from pooled human serum was administered intravenously on day 0 and day 8 to 6 month old APP-Tg mice that overexpress human Aβ (APPSwe/PS1ΔE9 mouse strain). A sustained increase of intact Aβ concentrations in peripheral blood determined was evident [Fig 3 below]. As the injected human IgM did not bind Aβ detectably, the evident increase of pepripheral Aβ is not due to peptide stabilization by formation of immune complexes. This suggests the feasibility of depleting brain Aβ as a consequence of peripheral IgM catalyzed Aβ hydrolysis.”(5)

Figure 3. Increase in peripheral Aβ in TG AD model mice following injection of human Aβ-targeting IgM. From (5)
Since the catalytic IgM pools did not form stable immune complexes, the conventional mechanisms for antibody-mediated efflux of Aβ from the CNS could not apply. Dr. Paul’s group proposed mechanism of this peripheral mobilization is thus distinct from that apparaently in place with sequestering IgG-based immunotherapies (active and passive), leading to anticipated therapeutic and safety advantages, as illustrated in Figure 2.
Aβ-targeting IgV clones remain in the early stages of development, placing it in a crowded field of other recently-developed anti-Aβ immunotherapies as candidates that appear to be approaching the exit spout justifiably draw the greatest amount of attention. But its novel, molecule-“autonomous” mechanism of action is more in line with the core principles of regenerative engineering, and in principle offers a high promise of therapeutic efficacy, attractiveness for preventive use in nondemented older adults, and little risk of adverse reactions known or anticipated in others far further developed. Their approach thus merits careful attention — and even cautious excitement — in its own right.
References
1. Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010 Feb;6(2):108-19. PubMed PMID: 20140000; PubMed Central PMCID: PMC2864089.
2. Paul S, Planque S, Nishiyama Y. Beneficial catalytic immunity to Abeta peptide. Rejuvenation Res. 2010 Apr-Jun;13(2-3):179-87. Review. PubMed PMID: 20370602.
3. Cribbs DH. Aß DNA vaccination for Alzheimer’s disease: focus on disease prevention. CNS Neurol Disord Drug Targets. 2010 Apr;9(2):207-16. PubMed PMID: 20205639.
4. Black RS, Sperling RA, Safirstein B, Motter RN, Pallay A, Nichols A, Grundman M. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2010 Apr-Jun;24(2):198-203. PubMed PMID: 20505438.
5. Taguchi H, Planque S, Nishiyama Y, Szabo P, Weksler ME, Friedland RP, Paul S. Catalytic antibodies to amyloid beta peptide in defense against Alzheimer disease. Autoimmun Rev. 2008 May;7(5):391-7. Epub 2008 Apr 9. Review. PubMed PMID: 18486927; PubMed Central PMCID: PMC2430036.
6. Paul S, Planque S, Nishiyama Y. Immunological origin and functional properties of catalytic autoantibodies to amyloid beta peptide. J Clin Immunol. 2010 May;30 Suppl 1:S43-9. PubMed PMID: 20454852.



