Short summary: When blood from a biologically aged animal is transfused into a young one, the young animal suffers “pro-aging” effects. A recent study shows that some of the “pro-aging” effects are derived from senescent cells in the old animal, and accordingly that blood from an old animal has less of a “pro-aging” effect if scientists remove some of the cellular damage (senescent cells) from the old animal before its blood is harvested. By contrast, reducing the burden of “pro-aging” signaling factors in old blood is not enough to remove existing damage from a similar-aged mouse’s biologically aged tissues. This contrast emphasizes the importance of directly removing aging damage if longevity therapeutics are to achieve durable and ongoing shifts toward youthful health.
Something remarkable happens when scientists surgically join a young and an old animal together such that they share each other’s circulatory system: for a short window of time, multiple functions in the aged animal are temporarily “rejuvenated.” First studied in the early 20th century, such “heterochronic parabiosis” was nearly forgotten until it was revived with a landmark 2005 paper by Irina Conboy, her life-and-science partner Mike Conboy, Thomas Rando, and others.
Less emphasized in most discussions of parabiosis is the flip side. Just as the old animal is exposed to the young animal’s blood and undergoes a temporary regenerative process, the young animal is equally exposed to the old parabiont’s blood, which makes the young animal functionally older across many of the same domains.
As we discussed in a previous post, the Conboys’ 2005 paper launched a new era of direct study of parabiosis itself — and it also drew a lot of serious and competent scientists into the search for the hypothetical “pro-youth” factors that these researchers assumed must be responsible for the sudden revitalization of the aged animal.
But from the very beginning, the Conboys emphasized that “pro-youth” factors in young animals’ blood are not the only possible explanation for the rejuvenating influence of parabiosis. The old animal’s blood is seething with high concentrations of “pro-aging” signaling factors, and when scientists conjoin the aged animal to a younger parabiont, half of the old animal’s blood is removed from its circulation and transferred to its young pairmate. And the one thing of which we can be confident is that the young animal’s blood has much less of these same “old blood” factors, and they’re under more youthful regulation.
Additionally, as the gunk present in the old animal’s blood passes through the young animal, its younger and healthier liver, kidneys, and other organs are better able to metabolize and excrete them before the blood recirculates to its aged mouse-of-origin.
For much of the time since that first landmark paper, the Conboys have been systematically investigating this alternative mechanism. And each experiment has piled on progressively more compelling evidence that such “dilution” of pro-aging factors is not only a critical component of the temporary rejuvenating effects of parabiosis, but is the whole enchilada.
Two studies have been especially important. In a 2015 study funded by SENS Research Foundation, the Conboys were able to exchange the blood of old and young animals directly, without passing the blood through the opposite-aged parabiont, thanks to a device engineered by SRF alumnus Justin Rebo and Keith Causey. The results showed that much of the apparent rejuvenating effect of young blood on old animals was actually the result of the aged animal’s blood being filtered and metabolized by the young parabiont’s organs rather than anything present in the young animal’s blood itself.
And then in late 2020, they published results that can only be described as shocking, even to those of us most skeptical of the existence or importance of “pro-youth” factors in young animals’ blood. In a bold and simple experiment, the Conboys showed that diluting an old animal’s plasma with mere saline solution and a biologically inert protein was enough to achieve rejuvenation equivalent to — and in some ways superior to — the classic stitch-and-share method of parabiosis.
The Conboys then conducted preliminary experiments that suggest that those animal experiments also have implications for aging in humans. Here they took advantage of an established plasma-dilution procedure in humans called therapeutic plasma exchange (TPE). Like the “neutral blood exchange” procedure used in the plasma dilution experiments in rodents, TPE is currently used to deplete high concentrations of undesired proteins, such as inflammatory proteins and autoimmune antibodies that cause pain and organ destruction in people with autoimmune diseases, or the atherosclerosis-driving lipoproteins apoB and lipoprotein (a) in people with genetic disorders that cause them to produce extraordinarily high levels of them.
Thus, the quest for “pro-youth” factors in the young animal’s blood now appears to be quixotic: instead of being a source of positive rejuvenating factors, the young blood emerges as a less-harmful “filler material” for the oppressive old blood it displaces.
In these experiments, mouse muscle stem cells were much more able to replicate themselves after being bathed in plasma that doctors had taken from aged people after they had undergone TPE than when the same cells were mired in “undiluted” old plasma from the same people. And more recently, the Conboys showed that after several rounds of TPE, plasma dilution in aged humans leads to an overall younger signaling profile, “including youthfully restored pro-regenerative, anticancer, and apoptotic [cell suicide] regulators”.
Where there’s Smoke, there’s Fire …
In one of their latest studies, the Conboys asked a kind of chicken-and-egg question. Does aging damage drive the pro-aging signaling environment in old plasma? Or does old plasma cause the aging damage? As is usually the case in the degenerative aging process, things turned out to be a vicious cycle of sorts — but turning the experiment on its head told us a lot about which one is in the driver’s seat.
To get a grip on this question, the Conboys chose to look at the interplay between circulating factors and senescent cells, because each of them is known to worsen the other. On the one hand, senescent cells are themselves a kind of aging damage; on the other hand, they release a complex mixture of inflammatory, growth-promoting, and structural protein-degrading enzymes collectively known as the SASP, which not only deranges its neighboring cells but also travels through the plasma, impacting cells widely across the entire body. And closing the vicious circle, one of the effects of the SASP is the conversion of healthy cells into senescent cells, in a phenomenon called “secondary senescence.”
The Conboys first asked the question: could “pro-aging” factors in blood from a biologically aged organism drive aging damage in a young animal? The answer: Yes. In line with previous work on secondary senescence, when they bathed cells from the deep layers of mouse skin in old mouse blood, the cells began to exhibit signs of senescence. The same thing happened when they similarly bathed some (but not all) human cell types in old human blood. Notably, the levels of two specific SASP factors in aged human plasma (interleukin-6 (IL-6) and the protein-degrading enzyme MMP-3) correlated with the level of IL-6 that human kidney cells subsequently released after the Conboys cultured the cells in old human plasma.
Again, this is exactly what other scientists (including those at SENS Research Foundation) have been reporting about the spread of senescence from senescent cells into normal, nonsenescent cells in laboratory studies. But would it hold up in living, breathing mice? Remarkably, even a single round of old blood transfusion was enough to push a substantial number of the cells in young mice into senescence. The effect emerged within the first two days, and even more senescent cells crept into the young animals’ tissues over the following two weeks. And the corrosive spread of senescent cell markers was accompanied by a surge of circulating SASP factors in the young animals’ blood.

Between the eruption of senescent cells in their tissues and the tainting of their blood with “pro-aging” factors, the young mice became afflicted with many of the infirmities of old age. The aged blood sapped them of strength and endurance, fat infiltrated their muscles, they suffered minor kidney damage, and their liver function declined as the organ became somewhat fibrotic.
But now we come to the second question, which is at the core of the entire heterochronic parabiosis phenomenon. Why does an animal’s blood become fouled with these baleful proteins with age? Some of the wilder speculations have postulated that this rise in inflammatory factors and damaged proteins is driven by a central aging program: a ticking time bomb lurking somewhere in our tissues that begins sending out the fateful command to age and die when the clock strikes the witching hour. That’s a highly unlikely explanation, since such programmed aging is incompatible with natural selection.
The main alternative hypothesis — one that aligns with what we know about evolution and the biological aging process — is that all of this deranged “pro-aging” signaling is the agonized biochemical crying-out of a body riddled with aging damage. As cells and essential biomolecules become damaged, they increasingly function abnormally, including in the production and reception of signaling molecules. Meanwhile, the body’s immune and repair systems go berserk in an increasingly futile and self-destructive attempt to repair or adapt to the rising and increasingly unrepaired number of dysfunctional units within it.
Within the scope of aging damage in our tissues, the burden of senescent cells — and because of it, their SASP — has long been established as a contributor to the molecular din of abnormal signal molecules in old blood. So the Conboys set out to probe the role of senescent cells in transforming an old animal’s blood into a “fountain of aging” in the most direct way possible. They would purge the tissues of aged mice of a significant number of their senescent cells using either of two different drug regimens that destroy senescent cells (“senolytics” ): the failed cancer drug Navitoclax or the cocktail of the cancer drug dasatinib plus the plant phenolic quercetin (D+Q). Then they would see if having removed some of that damage from the old animals’ tissues would make their blood less “pro-aging.”
And that’s exactly what happened. Pretreating an old mouse with either senolytic approach before transferring its blood into a young mouse greatly reduced the “pro-aging” effects of its blood on the young animal, driving fewer of the young mouse’s cells into senescence and causing less organ dysfunction. However, such pretreatment didn’t abolish the “pro-aging” effects of the old mouse blood entirely: most notably, the young recipient mouse muscles’ ability to repair themselves remained just as impaired after being subjected to blood from old senolytic-pretreated mice as it had been after taking on board the blood of old mice that had not been thus treated.

This result was as you would predict from what we know about the role of senescent cells in aging. Aging bodies accumulate senescent cells for a variety of reasons, and those cells secrete the SASP into the surrounding tissues, including the blood — and SASP factors drive other, non-senescent cells into senescence. In turn, more senescent cells lead to more age-related disease and dysfunction. So cutting down the concentration of SASP factors in old blood by destroying the cellular factories that produce them will in turn cause less harm when a young mouse is subjected to it.
… But Air Purifiers are Not Fire Extinguishers
What we’ve learned so far is what happens when signaling factors from damaged cells are transferred, via the blood, into another animal that has few senescent cells of its own. Damage (senescent cells) begets more damage (secondary senescent cells) and tissue dysfunction (seen in muscle, kidney, and liver after old-to-young blood transfer). And, of course, the secondary senescent cells induced in the young animal will produce SASP factors of their own, perpetuating the vicious cycle that makes the survival rate of aging animals (human or otherwise) plunge into an exponential descent once they reach their untreated middle age.
If pretreating old blood-donor mice with senolytics greatly reduces the number of new senescent cells that erupt in the tissues of young mice following a transfusion of old blood, what kind of benefit might old mice that had not received senolytics get if scientists replaced some of their own old blood with the lower-SASP blood of other old mice that had benefitted from a senolytic sweep? The Conboys tried this — and there was more or less no effect! That’s right: no effect on senescent cell burden; no reduction in circulating SASP factors; and no improvement in muscle strength, physical endurance, kidney damage, or liver fibrosis.
Why would this be? Why would replacing a mouse’s own pro-aging blood with blood that is less pro-aging due to a lower concentration of SASP factors so greatly blunt the damage done to a young animal but have essentially no effect on an old animal? The most likely answer more or less boils down to: because the recipient animal is old — meaning, by definition, that its tissues are already riddled with aging damage.
Remember: degenerative aging occurs over time in an animal not because of the simple passage of birthdays or the ticking of an inbuilt “death clock,” but because cellular and molecular damage accumulates over time in an organism’s tissues. As the side effects of metabolism mangle more and more of the functional units in an aging organism’s tissues, those tissues’ functions become more and more impaired. And when the number of remaining functional units has fallen below a critical threshold, we label the resulting tissue-specific dysfunction a “disease” of aging.
So when a young animal’s tissues are subjected to the deranging biochemical denial-of-service attack of an old animal’s blood, the relative rise in its overall burden of damage and dysfunction is high precisely because its baseline level of damage and dysfunction is low.
And in turn, the mitigating effect of partially damping down the signaling chaos of an old animal’s blood by replacing it with lower-SASP old blood is relatively small. The pre-existing burden of damage and dysfunction in an old animal’s tissues is already so great that simply reducing the amount of deranging signals in its circulation doesn’t do much to lower its total damage level or to improve its health, in part because there are so few functional units left available to damage.
As an analogy, consider the recent student loan forgiveness initiative implemented by the White House. Most students with existing student debt had $10,000 taken off their balance. That same $10k can mean quite different things to different borrowers, depending on how much debt they were carrying in the first place. To a person who is only $15,000 in debt, that $10k can have transformative effects on their monthly budget, and bring the finish line close enough that they can suddenly see the end of it. But a person who is already struggling under $200,000 of student debt may barely notice that anything has changed when their servicer trims $10k off of their total.
The second reason why replacing some of an old mouse’s blood with blood from an old senolytically-pretreated donor mouse has next to no effect is the reciprocal of what we just went over. The fact that an old mouse (or human) has suffered so much more damage to its cellular and molecular structures than a young organism does means that it has far fewer intact units left behind to carry out the essential functions of the tissue.
Thus, because a young animal can draw on a generous reserve of intact cells and biomolecules to buffer the effect of an old animal’s blood, it can take better advantage of a modest reduction in the suffocating signaling of an old blood transfusion. By contrast, the same small reduction in the “pro-aging” signaling environment has next to no impact on an old animal because it has so few remaining functional units left. It’s like removing the straw from a camel whose back is already broken. Only when scientists draw down the oppressive systemic factors much more dramatically via a young blood transfusion or TPE can the relatively few remaining undamaged cells and biomolecules in an old organism’s tissues rally enough to add up to a measurable improvement in function.
This may also be part of the reason why — despite the many rejuvenation-like effects of both young-to-old parabiosis and TPE — a three-month-long regimen of parabiosis with a younger mouse has no effect on an old mouse’s lifespan, even though exposure to old blood and organs during the same period decimates a young animal’s life. The old mouse’s tissues may function a bit better when the dire murk of aging blood no longer suffocates them, but without actually repairing the cellular and molecular damage already in their tissues, their few surviving functional units can’t hold out for long, no matter how much scientists improve the signaling environment around them.
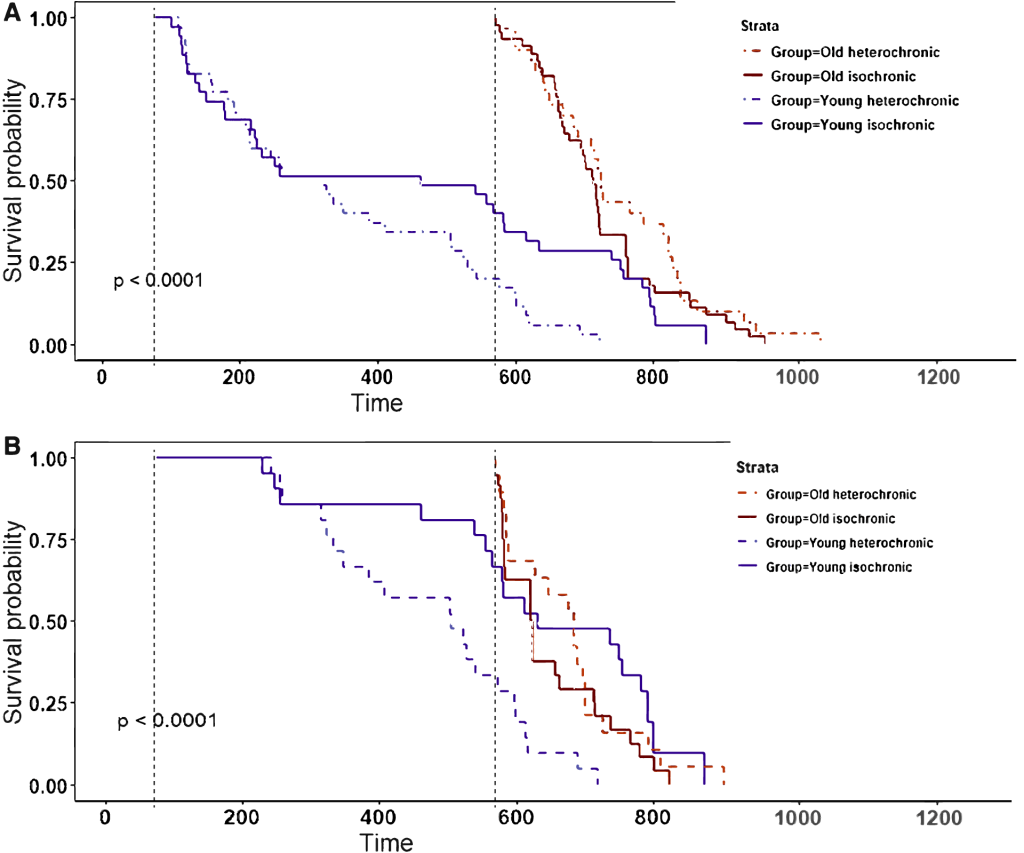
You might wonder if the failure of parabiosis with a young animal to extend the life of an old one could have been an artifact of the way scientists perform conventional parabiosis. True parabiosis is quite the ordeal: there are the direct risks associated with the surgical procedure required to join the animals’ circulatory system, and then there is the stress experienced by a mouse who spends months of its life literally joined at the hip with another animal as it eats, sleeps, and goes to the bathroom. You might therefore imagine that these challenges and stressors might have overwhelmed the anti-aging benefit that the old animals would otherwise have gotten from partially replacing their suppressive old blood with the less-suppressive blood of much younger animals.
However, the researchers who conducted the lifespan study largely accounted for such effects by doing an additional analysis that looked only at the subset of mice that survived at least 150 days after being separated from their young parabiont. At that point, the animals should largely have recovered from the effects of such “parabiosis disease” — but this subgroup analysis did not fundamentally change the results (compare (a) and (b) in the Figure above). Whichever way you look at it, young-to-old parabiosis may have afforded small bumps where the survival curves had separated, but it failed to deliver any consistent or sustained effect of the sort you see with Calorie restriction or rapamycin treatment. Certainly it produced nothing equivalent to the devastating effects of exposing young animals to old animals’ blood over the same time period. Moreover, an earlier experiment had shown that the minimally-invasive approach of weekly injections of young plasma for a full 16 months didn’t nudge the survival curve either.

Don’t Fertilize the Weeds
In addition to the sheer burden of pre-existing aging damage in the old animal that young-to-old blood transfusion or parabiosis cannot undo, another possible reason why the short-term regenerative burst of these procedures has so little durable effect is that are potential downsides to restoring a signaling system typical of a young animal in an animal that has already suffered substantial structural damage due to aging. One potential example is how to the short-term boost in regeneration that comes with them might fuel the growth of precancerous lesions or worsen fibrosis.
There were no necropsies of the animals in the parabiosis lifespan studies we just discussed to determine their cause of death, so we don’t know whether these diseases were worsened in the old mice parabiosed to young ones or given young plasma. What lends the idea biological plausibility is the curious fact that while the incidence of most diseases of aging rises steadily (or even exponentially) with age, cancer seems to level off or even decline toward the end of a current lifespan (see the Figure below).
Scientists have also shown experimentally that something about biological aging eventually makes our tissues less accommodating to cancers. For instance, when you infect mice with a virus that can cause new cancers to form, tumors are actually less likely to emerge in an old mouse than in a young one — and the tumors that do form grow more slowly. This sluggish growth is all the more surprising when you consider the older organism’s weakened immune system, which limits its ability to kill the virus-infected and/or precancerous cells.

This is one of the paradoxical benefits of the suppressive signaling environment in an aging organism: even though more cells are accumulating the mutations that would otherwise drive them to become cancerous, the aging body no longer provides the growth factors and other signals that would be required to support their lethal multiplication. Such pro-growth factors include the sex hormones that drive estrogen receptor-positive breast cancers and testosterone for most forms of prostate cancer. Also, the aging body grows new blood cells (angiogenesis) more slowly due to declining levels of VEGF and related signaling molecules. This feeble angiogenesis makes it hard for the aging body to recover from a heart attack or stroke, but it also makes it harder for emerging cancers to develop the blood supply they need to fuel their furious growth.
In a third example, a great deal of research suggests that brain-derived neurotrophic factor (BDNF), a critical molecule for the growth and development of neurons, protects us against cognitive decline, post-traumatic stress disorder, and depression. Yet it too is hijacked by cancer. So while the age-related decline in BDNF makes us vulnerable to age-related cognitive decline and mood disorders, it might also make the aging organism’s tissues less hospitable to cancer.
So when an old animal regains some of the pro-growth signaling of a younger animal through young-to-old parabiosis or TPE, the short-term regenerative benefits may come with a dark side if it is carried on for longer periods or with repeated treatment cycles.
Young Tissues Send Young Signals
The way to escape such dilemmas is to tear them out at the root. The deranging signaling environment in an old organism is like the sludgy old oil lubricating the damaged components of a poorly-maintained vehicle. Oil breaks down more quickly in a damaged engine, and as the oil becomes more viscous and laden with impurities, it in turn inflicts further damage on the interfacing parts of the machine. An oil change (parabiosis or TPE) will make the damaged engine run more smoothly, and will cause less additional damage to the engine damage going forward than continuing to drive the car with old, increasingly degraded lubricant. But a simple oil change does nothing to address the damage that the engine has already suffered after years on the road without maintenance. To do that, you must remove, repair, and replace the damaged components themselves.
That’s precisely the strategic target of the SENS approach to longevity therapeutics. When you finally take your car into the shop to replace its worn-out parts, the engine will run as smoothly as it did the day you first brought it home. And not only will the engine itself be in peak condition, but the “lubricant” through which the parts interact. That’s because, unlike in a manufactured machine like a car, the fluids through which the components of a living organism interact (the signaling environment in the blood) are not externally-added lubricants but a product of the organism itself. Young animals (including young human animals) have young signaling environments because they have a low burden of aging damage: few senescent cells throw out SASP; few cells overtaken by deletion-mutation-bearing mitochondria spread oxidative stress across the body; few amyloids trigger inflammation in the brain; few atherosclerotic foam cells recruit inflammatory T-cells into our arteries; and so on.
By removing, replacing, and repairing the damaged cellular and molecular units that comprise our tissues, tissues will resume functioning youthfully, including the signaling molecules they send out, transmit, and respond to. The signaling environment will be youthful because the organism will be youthful, and from that rejuvenated structure and signaling will flow robust health and vitality for as long as we continue to maintain this most precious of all machines.