The intrinsic biological aging process is driven by the accumulation of damage to the cellular and molecular structures in tissues and organs, resulting from the biochemical side-effects of essential metabolic processes. In turn, this rising decay of cellular and molecular structures drives the age-related rise in disability, disease, dependence, dementia, and ultimate risk of death. Because of this well-established connection, and because of the methodological difficulties in evaluating the effects of interventions on the extension of the youthful health and functionality of model organisms, the ability of an intervention to extend life in well-husbanded, nonobese, longevous strains of laboratory animals remains a necessary if surrogate metric for evaluating the potential of interventions to maintain or restore the health of aging organisms, including humans.
To date, very few interventions have been found to meet this rigorous standard — and most of them involve germline mutations, of no likely human translatability. The most robustly-documented environmental manipulation that extends life and health in mammals remains Calorie restriction (CR), and this has led to a strong interest in the biogerontology community in evaluating pharmacological agents that might might provide its benefits without requiring the arduous adoption of a CR diet in humans — so-called “CR mimetics.”(1) In an earlier post, we reviewed a long list of putative CR mimetics that have failed in lifespan studies. Amongst these was the phytoalexin polyphenol resveratrol, famously found in trace amounts in wine and widely anticipated to be one of the first effective life-extending, youth-preserving compound, but found ineffective in testing in nonobese, longevous mice.(2) We also reviewed the results of the serendipitous late-life lifespan study of rapamycin (sirolimus/Rapamune®), an inhibitor of the mammalian Target of Rapamycin (mTOR) pathway, through the NIA’s Interventions Testing Program (ITP), “a multi-institutional study investigating treatments with the potential to extend lifespan and delay disease and dysfunction in mice.” This study was hailed as a breakthrough, being the first robust demonstration of lifespan extension in mammals by a pharmacological agent, although as we reviewed, the absolute effect of rapamycin was limited, and in fact not entirely clear in males.(3)
The ITP has now provided important confirmation of these findings, by testing resveratrol at higher doses using more robust animal models, and rapamycin beginning in much younger adult mice.
Rapamycin was administered in food to genetically heterogeneous mice from the age of 9 months and produced significant increases in life span, including maximum life span, at each of three test sites. … Rapamycin was found to lead to improved survival in both males and females when pooling across test sites, and to significant effects at each test site considered separately. … For males, rapamycin led to an increase of 10% in median age, averaged across the three sites, and an increase of 16% in the 90th percentile age [ie, by operational definition, maximum lifespan -MR]. For females, the corresponding values were 18% for median, and 13% for 90th percentile ages. … Rapamycin attenuated age-associated decline in spontaneous activity in males but not in females. Causes of death were similar in control and rapamycin-treated mice.(4)
The results are important on several fronts. The activity test gives some preliminary additional evidence of extended “healthspan” in rapamycin-administered mice. Similarly, the lack of a differential effect on specific causes of death suggests again that the life extension effects of rapamycin are the result of a far-reaching improvement in all the animals’ systems, and not due to inhibiting some single, and perhaps idiosyncratic, overriding cause of death. The confirmation of the effect in males was important, as it had been ambiguous in the earlier study, thanks to a surprising trend toward an early survival advantage in treated males prior to the onset of intervention, and the lack of any clear effect at one of the 3 testing centers (The Jackson Laboratories).
The actual lifespan results, however, were also revealing, and in a surprising way:
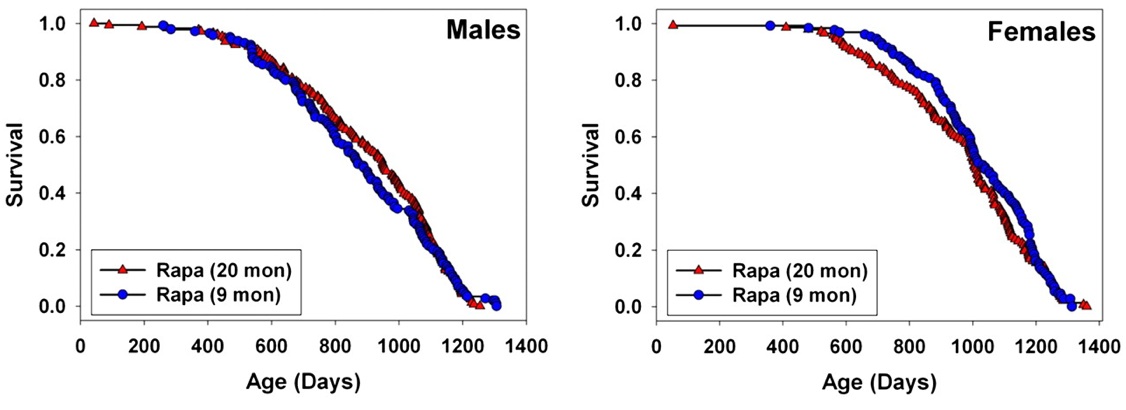
Figure 1. Overlapping survival curves from rapamycin administered in early or late adulthood in mice. From (4)
[This figure] a graphical comparison between the results of the current study [with rapamycin administered from age 9 mo onward (4)], and the results of the study comparing mice exposed to rapamycin from 20 months of age [(3)]. The two data sets were not produced simultaneously, but they do represent work done using the same conditions of drug preparation, diet, water source, housing, and genetic stocks at the same three sites, with only a 1 year lag between start dates. For male mice, starting rapamycin at 9 months rather than at 20 months did not lead to any improvement in survival [relative to the later age of onset]; the two cohorts were not significantly different by log-rank test, at p = .74. For female mice, there is a suggestion that earlier exposure to rapamycin may have led to some slight decline in mortality risk before ∼1000 days of age, but the log-rank test is not significant (p = 0.31). The Wilcoxon–Breslow test, which does not assume that the difference in risk between the test groups is constant at all ages and which gives more weight to earlier deaths, was also nonsignificant for females, at p = 0.09 (two tailed). … In principle, however, an agent that slowed the overall process of aging would be expected to have a stronger effect if started at a relatively early age. [This is because it would be expected that the greater duration of treatment would prevent a larger net burden of aging damage to accrue, leading to greater lifespan benefit, as is observed eg. in CR administered at weaning, in middle age, and toward the end of the “natural” lifespan (5) -MR] Thus, if later work confirms that rapamycin treatment starting in later life is fully as effective as treatment started early in adult life, this could be taken as evidence that the agent is not modulating aging so much as limiting the pace of, or vulnerability to, late life illnesses, such as the neoplastic diseases that lead to most of the deaths in UM-HET3 mice.(4)
Alternatively, it might suggest that the intervention is not interfering with the underlying structural decay of aging, but in some of its downstream metabolic sequelae, affording treated animals a greater ability to withstand the pathological systemic effects of aging damage once the burden is sufficient to exert a systemic, mortality-accelerating influence. Such an effect was suggested in an earlier, not fully convincing report of similar life extension in mice were administered the spin trap antioxidant, N-tert-butyl-alpha-phenylnitrone (PBN), at ages 18.5, 21.5, or 24.5 mo.(6) This is also consistent with the implications of observations of systemic effects of aging on stem cells(7,8) and of the antioxidant effects of R-lipoic acid when administered to young, but not to old, rodents,(9) and possibly with the effects of cognitive engagement in delaying the onset of clinical dementia but accelerating its course.(10-12)
The confirmation of a lack of effect on lifespan at higher doses of resveratrol was equally important in its own way, granted its high prominence and its ongoing fueling of sales and unscrupulous promotional tactics in the weakly-regulated dietary supplement market:
Resveratrol (at 300 and 1200 ppm food) and simvastatin (12 and 120 ppm) did not have significant effects on survival in male or female mice. … Our resveratrol data thus serve to confirm the absence of any effect of this agent on mouse life span, using doses two- to eightfold higher than the dose studied by Pearson and colleagues [(2)] and using genetically heterogeneous mice of both sexes rather than male C57BL/6Nia mice alone. (4)
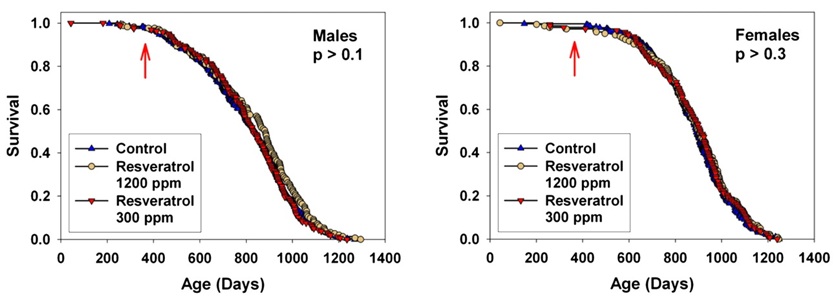
Figure 2. Resveratrol Fails to Extend Life in Normal, Genetically-Heterogeneous Mice. From (4)
This latter point is noteworthy, not only because of the a priori reasoning that a study population with a wider genetic diversity is less likely to lead to artifacts due to a strong effect on some strain-specific, life-limiting disease, but because of the specific concern that the authors of the earlier report had raised:
resveratrol treatment did not significantly alter the distribution of pathologies in [standard diet] groups. This included neoplasias, despite the potency of resveratrol against implanted or chemically induced tumors … This may be related to the fact that the vast majority of these cases were lymphomas, a tumor type for which the efficacy of resveratrol has not been thoroughly assessed, and that is thought to be triggered mainly by endogenous retroviruses in mice (2)
Combined with their positive results with rapamycin, the failure of resveratrol to extend life using resveratrol in normal mice over a very wide range of doses should reasonably be taken to put the resveratrol “story” to test.
On the other hand, the ability of rapamycin to extend life in these mice has been confirmed, and expanded to a preliminary extent. Naturally, further studies are underway or proposed to elucidate the full nature of these effects:
One such study will evaluate doses of rapamycin both higher and lower than the dose used for the initial longevity studies … Cross-sectional histopathology will help to identify earlier stages of common age-dependent illnesses, including those that do not typically lead to death …Studies of multiple age-dependent physiological outcomes will also be included. These … will help to show whether the life span improvement represents merely a global inhibition of neoplastic disease, or rather an authentic antiaging effect that includes anticancer protection as just one among many consequences. The follow-up studies will also evaluate biochemical changes in multiple tissues, to see which tissues show long-term and short-term inhibition of mTOR and modulation of mTOR-dependent cellular feedback circuitry. … It is equally plausible that the health benefits of rapamycin principally reflect a change in one or a few cell types, perhaps located in hypothalamus, vascular endothelium, a hormone-producing cell type, or a stem cell compartment, whose beneficial effects on other cell types are independent of the level of mTOR function in the downstream target tissues. Evaluation of rapamycin effects on rodent models of specific diseases is likely to be very informative in this regard. For example, two recent studies have evaluated rapamycin effects on different mouse models of Alzheimer’s disease … Each study found that rapamycin rescued memory deficits and slowed pathological manifestations in these neurodegenerative models. Analysis of effects on other rodent (and perhaps canine) models of late-life diseases should prove equally informative. It will also be helpful to learn if rapamycin can further improve the longevity benefits produced by specific mutations or diets or other drugs. Researchers have learned much about the biology of aging by studies of caloric restriction and of antiaging mutations, and the advent of effective antiaging drugs should provide additional leverage for new work on the factors that time the coordinated appearance of age-related decline. In addition, work on antiaging interventions holds more promise for the eventual development of protective medicines than approaches that entail modification of germline genes or lifelong compliance with extreme dietary restrictions.(4)
At the same time, whatever these studies may reveal, even the most optimistic reading of these results and an assumption of perfect human translatability is still overshadowed by how limited the results are. Interventions such as rapamycin, which only retard the rate at which aging damage accumulates (or, perhaps, allows the organism to carry on functioning for a longer period of time under its accumulating burden), can only temporarily delay the onset of age-related ill-health, not arrest or reverse it — and in the case of rapamycin, the first pharmacological agent to extend the lives of otherwise-healthy mammals, its ability to do even this has been found to be limited. Rejuvenation biotechnology offers an alternative approach, and the promise of a life of greatly-extended youthful functionality.
References
1: Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci. 2010 Jul;65(7):695-703. Epub 2010 Apr 6. Review. PubMed PMID: 20371545; PubMed Central PMCID: PMC2884086.
2: Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008 Aug;8(2):157-68. PMID: 18599363 [PubMed – as supplied by publisher]
3: Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009 Jul 16;460(7253):392-5. Epub 2009 Jul 8. PubMed PMID: 19587680; PubMed Central PMCID: PMC2786175.
4: Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, But Not Resveratrol or Simvastatin, Extends Life Span of Genetically Heterogeneous Mice. J Gerontol A Biol Sci Med Sci. 2010 Oct 25. [Epub ahead of print] PubMed PMID: 20974732.
5: Rae M. It’s never too late: calorie restriction is effective in older mammals. Rejuvenation Res. 2004 Spring;7(1):3-8. Review. PubMed PMID: 15256039.
6: Saito K, Yoshioka H, Cutler RG. A spin trap, N-tert-butyl-alpha-phenylnitrone extends the life span of mice. Biosci Biotechnol Biochem. 1998 Apr;62(4):792-4. PubMed PMID: 9614711.
7: Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005 Feb 17;433(7027):760-4. PMID: 15716955
8: Mayack SR, Shadrach JL, Kim FS, Wagers AJ. Systemic signals regulate ageing and rejuvenation of blood stem cell niche. Nature. 2010 Jan 28;463(7280):495-500. PubMed PMID: 20110993.
9: Hagen TM, Ingersoll RT, Lykkesfeldt J, Liu J, Wehr CM, Vinarsky V, Bartholomew JC, Ames AB. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999 Feb;13(2):411-8. PubMed PMID: 9973329.
10: Wilson RS, Barnes LL, Aggarwal NT, Boyle PA, Hebert LE, Mendes de Leon CF, Evans DA. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology. 2010 Sep 14;75(11):990-6. Epub 2010 Sep 1. PubMed PMID: 20811001; PubMed Central PMCID: PMC2942032.
11: Chaves ML, Camozzato AL, Köhler C, Kaye J. Predictors of the progression of dementia severity in brazilian patients with Alzheimer’s disease and vascular dementia. Int J Alzheimers Dis. 2010 Mar 14;2010. pii: 673581. PubMed PMID: 20798750; PubMed Central PMCID: PMC2925083.
12: Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006 Mar;77(3):308-16. PubMed PMID: 16484637; PubMed Central PMCID: PMC2077720.



