
SENSible Question: In addition to allotopic expression (AE), SRF has begun other projects targeting mitochondrial dysfunction; could you elaborate on those and how they relate to the original strategy of allotopic expression?
Right. Mitochondrial mutations — and above all, large deletions in the mitochondrial DNA — accumulate in long-lived cells over our lifetime. And until we can do something to repair or bypass that problem, the overtaking of this small fraction of our cells by deletion-bearing mitochondria will continue to drive diseases of aging that include Alzheimer’s and Parkinson’s diseases, the loss of functioning muscle fibers and strength with age, and other diseases and disabilities of aging.
Long before there was a SENS Research Foundation — even before a “Strategies for Engineered Negligible Senescence” (SENS) platform existed — our founding CSO Dr. Aubrey de Grey surveyed the possible solutions for this problem, and the only one that seemed viable was allotopic expression (AE): engineering “backup copies” of the mutation-prone mitochondrial genes into the safe harbor of the nucleus. AE wouldn’t prevent large deletions in mitochondrial DNA from happening, and it wouldn’t repair them either — but it would effect a functional fix, because it would allow our mitochondria to sustain or restore normal energy production even if the original mitochondrial genes suffered disabling mutations. And when SENS Research Foundation was established, the very first team of in-house scientists we recruited was put to work on allotopic expression as a MitoSENS strategy.
So why — after making great leaps forward with the science — are we now breaking ground on entirely new MitoSENS strategies? A few reasons. Considered at the most fundamental level, AE itself is an inherently difficult biotechnological challenge. Then there’s the additional hurdle of delivering it to those cells that are vulnerable to mitochondrial mutations with age. That alone is enough reason to keep an eye out for alternative ways to deal with mitochondrial mutations: success is not guaranteed, and putting together all the necessary pieces will take time. So it’s good to keep one’s eyes peeled for solutions that might be easier or faster to implement, granted that our future lives and health are at stake.
And we’ve always noted that it’s good to have options: Dr. de Grey and I dedicated much of Chapter 6 of Ending Aging to potential alternatives to AE. And as we’ll see, the alternative solutions that our MitoSENS scientists are working on now might not be so much alternatives to AE as complementary or even synergistic solutions, each with strengths and weaknesses and each deployed on different timescales.
Dr. Boominatham and colleagues in our MitoSENS lab are now working on two of these alternative strategies — both of them also thought up or endorsed by Dr. de Grey. You might think of them as backup strategies for the backup copies.
“Prescribed Burns” for Mitochondrial Transplantation
As discussed on our main page on mitochondrial mutations, one of these strategies is to use a form of mitochondrial transplantation to replace the cell’s mutation-bearing mitochondria with healthy ones. As you may have heard, clinician-scientists have been harvesting mitochondria from patients’ muscles and transplanting them into other tissues as an experimental therapy for a number of acute, emergency conditions — most notably for the reintroduction of oxygen assaults the hearts of young children can experience after surgery for congenital heart conditions. This “ischemia-reperfusion injury” depletes the cell of cellular energy (ATP), damages the mitochondria, and can lead to the death of heart muscle cells — and even the patient. When doctors transplant pre-harvested mitochondria into the heart during surgery, the transplanted cellular power plants enter the heart muscle cells and give them the quick boost of ATP they need to make a better recovery.
But these transplanted mitochondria don’t last long: once the crisis is over, tracer studies show that the cell’s surviving native mitochondria soon replace the newcomers by reproducing themselves. That’s absolutely fine when the cell only needs a few extra functional mitochondria for a temporary boost to get it through an acute emergency. But it obviously won’t help with the long-term problem we face in aging, where the cells’ own mitochondria are the bad actors. In that case, mutation-bearing mitochondria would quickly drive out the good, just as they do once the first large deletion mutation occurs in a cell during aging.
So for mitochondrial transplantation to work as a rejuvenation biotechnology, we need a way not only to get the transplanted mitochondria into the cells, but to enable them to bypass the selective advantage of the native mitochondria, and especially of the powerful advantage of mutation-bearing mitochondria.
This is where the relatively new biotechnology of “gene drives” come in. Instead of just introducing healthy mitochondria and hoping that they somehow take over the cell, we arm the mitochondria for transplantation with a genetic buzzsaw called a restriction enzyme. Restriction enzymes are molecular tools produced by bacteria to chop hostile DNA molecules up into fragments at or near defined target sites — sites known, appropriately enough, as restriction sites.
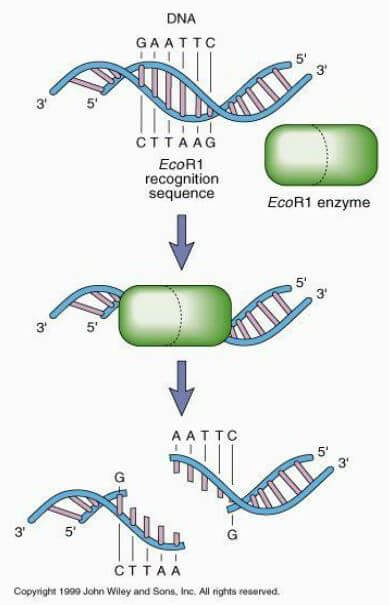
The name “restriction enzyme” comes from the fact that natural restriction enzymes are used by bacteria to “restrict” the growth of viruses that attack them by destroying their genetic material. Readers will be familiar with a couple of specific types of restriction enzymes that have already been harnessed for biotechnological use: the genome editing tools CRISPR/Cas9 and TALENS.
In the gene drive strategy, engineered mitochondria would use restriction enzymes like the weapons they originally were. The restriction enzyme with which mitochondria for transplant will be engineered will be designed to target one of the several restriction sites that are naturally present in the host’s mitochondria. And to prevent the transplanted mitochondria from dying by their own molecular swords, scientists would engineer the target restriction site out of their genomes, making them untouchable by the enzyme. Once they are taken up by the host cell, the engineered mitochondria would intermingle with the cell’s native, potentially mutant mitochondrial genomes. Then the restriction enzyme would quickly go to work eviscerating the cell’s original mitochondrial DNA, thereby clearing space to allow the new, transplanted mitochondria to take over.

“Now Let’s See Who You Really Are!”
(With apologies to Scooby-Doo and the Mystery Machine).
Remember that the reason why deletion-bearing mitochondria take over the cell is not because of runaway self-replication, but because they are nearly invisible to the machinery that detects defective mitochondria and tags them for destruction (“mitophagy”). The result is that while other mitochondria are periodically damaged and degraded during the course of normal operation, the Angel of Death passes over the deletion-bearing mitochondria. After multiple rounds of being spared while their genetically-intact fellows get culled, deletion-bearing mitochondria are the only kind left in the cell.
We can’t say much about the second strategy the MitoSENS team is exploring because it’s a very early-stage project, and we want to be sure we’re on the right track before making any announcements. All we’ll say for now is that our scientists have identified a drug that may potentially “unmask” deletion-bearing mitochondria, attracting the attention of the mitophagy machinery and allowing it to cull them. Under some circumstances, such “unmasking” is sufficient to keep deletion-bearing mitochondria at bay when they haven’t yet overtaken the cell. If the drug we’re testing (or a similar one) could do that, we might be able to keep many cells operating normally by holding deletion-bearing mitochondria down to a minority of the population, and keep other cells free of deletion-bearing mitochondria entirely by catching the first one and sending it to its grave.
Flipping Both Scripts
In a sense, the gene drive strategy is almost the reverse of AE. In AE, we engineer the nuclear DNA so that it can throw a life jacket to the mutation-bearing mitochondria inside the cell, delivering essential working proteins to keep them producing energy normally despite having suffered deletion mutations. In the gene drive strategy, we engineer the mitochondrial DNA of transplanted organelles outside the cell — and instead of engineering them to keep deletion-bearing mitochondria humming, we give them the power to purge the mutant mitochondria from the cell entirely.
The “unmasking” approach takes an angle that is completely different from both AE and the gene drive. Those strategies take for granted the fact that deletion-bearing mitochondria will overtake some cells as a result of aging processes, and either prop up the mutant mitochondria’s function (AE) or wipe them out and replace them (gene drive). The “unmasking” approach can only work before the takeover is complete, and helps the cell recognize deletion-bearing mitochondria for the threat they are so that they don’t achieve the wall-to-wall domination that makes them a threat to the cell and its neighbors in the first place.
The Swiss Cheese Defense
All of these approaches have potential advantages and disadvantages, and like layering multiple slices of Swiss cheese on top of each other, combining them may let one method cover the holes left by another so that we ramp up solutions for the problem of mitochondrial mutations faster and make the eventual solution more robust.
Because it puts the solution permanently in the cell’s nuclear DNA and works regardless of the mutation status of the mitochondria themselves, allotopic expression would create a robust and extremely durable fix. On the other hand, it is very technically challenging in itself, and delivery will also not be trivial — especially at the “last mile,” where the ultimate goal is to have coverage for every durable cell in the body.
Additionally, you can at least imagine a day when people have lived so long that a much larger fraction of the cells in a tissue have suffered deletion mutations than has ever occurred during our “natural” lifespans. Such cells will only be able to keep going because of nuclear “backup copies,” and with so many cells running on a kind of backup power, the function of the tissue may begin to get sluggish. At that point, wholesale replacement of the mitochondria themselves might become necessary even though it won’t be needed to reach longevity escape velocity.
At the other extreme, the “unmasking” approach is potentially the fastest path to a mitochondrial mutation longevity therapeutic of all of them, since drugs are easy to synthesize, test, and deliver. And most of them can easily reach broadly and deeply into nearly every tissue in the body and be administered continuously for extended periods of time, so the challenges of gene therapy or hyperlocal delivery of transplanted mitochondria just don’t apply.
We can expect such a drug to share two downsides with other “messing-with-metabolism” approaches. First, it would only delay (rather than arrest or durably reverse) the emergence of cells overtaken by mitochondrial mutations, so even if it held the enemy at bay for a few years or even a decade, any given cell would still need a rescue treatment with direct “damage-repair” therapies sooner or later. And second, the cellular machinery that such a drug would boost or inhibit is there for a reason, and it’s not to give refuge to mutant mitochondria. So even if (as our scientists hypothesize) interfering with the normal regulation of this machinery may help our cells avoid a hostile takeover by deletion-bearing mitochondria, it’s also bound to have side effects dictated by having the evolved purpose of this machinery not working as it is normally tuned to do.
The gene drive approach is somewhere in between. It might be faster to develop than AE, despite the SRF MitoSENS team’s substantial head start with allotopic expression. It also doesn’t have some of the specific delivery problems related to gene therapy as AE does. And in principle, it should solve the problem more directly than AE does, eradicating any mutant mitochondria and replacing them with healthy ones. On the other hand, it has its own delivery challenges. Mitochondria might have to be painstakingly transplanted right up close to hundreds of small segments of tissue spread across the body. And we don’t yet really understand how exactly transplanted mitochondria get into target cells, let alone whether some cell types might be resistant to taking them in — and if so, how we might overcome that problem. And we don’t yet have proof-of-concept of the critical step: using an encoded endonuclease to do a Texas Chainsaw Massacre on the original mitochondrial population.
So supposing that all three approaches work as conceived, you can imagine a scenario in which we use each of them in complementary ways. If (as is reasonable to assume) the “unmasking” drug proves to be the fastest to develop, we would deploy it first and buy people time while we keep working to develop the other MitoSENS therapies. Then we would deploy whichever of AE and the gene drive were ready to go first. There could even be different answers to whether either approach is ready depending on the tissue, with some tissues being easier to access with one or the other. And we might still rely on the “unmasking” drug as we worked to get full-tissue coverage with each of the other two.
Any stem cells and engineered tissues we deliver could be pre-engineered with AE. On the other hand, the gene drive could also be deployed to the existing cells in the same tissue in which those stem cells were being delivered to help minimize tissue damage from the surgery itself. Doing so would also take advantage of the tissues already being exposed by the surgeon’s knife, like when wise city planners take advantage of the street being dug up for sewer maintenance work to lay down underground electrical wiring.
If all the above were to work, we might ultimately transition to a combination of keeping things humming along in the background via the more or less permanent engineering of the nuclear DNA with AE, along with periodic hard resets of the mitochondrial population itself using the gene drive.
For now, all of this is dreams for our future. Today, our scientists are hard at work on the basic and translational science of AE, and on the more preliminary work on the gene drive and the “unmasking” drug (or a similar molecule) to determine if they are even worth pursuing. But the dream of a future beyond aging is what got us all here, and what will carry us forward into making those dreams our future reality.



