Short summary: UNITY Biotechnology was the first senolytic startup out of the gate to reach aging human subjects, and the failure of its Phase II trial in osteoarthritis was a crushing disappointment. A careful look at the underlying science and at three new scientific papers sheds light on the limitations of the trial and suggests ways to move forward.
Rooting dysfunctional cells out of aging tissues (ApoptoSENS) is an essential plank in the SENS platform of rejuvenation biotechnologies, and at the top of the ApoptoSENS most wanted list are senescent cells. Ablating senescent cells has also arguably been the standout early success story for the SENS “damage-repair” approach, and could easily have been the first rejuvenation biotechnology to be FDA-approved.
Instead, the first head-on punch against the damage of aging in humans came with the first FDA-approved AmyloSENS therapy: lecanemab (Leqembi®), a monoclonal antibody targeting beta-amyloid fibrils. Its Phase III clinical trial proved that it could put the brakes on the terrible downward trajectory of the disease, and it’s finally available to people suffering from neurodegenerative aging of the Alzheimer’s type and their families.
But anyone reading about the sweeping “anti-aging” effects of destroying senescent cells with “senolytic” drugs in aging animals and animal models of age-related disease could easily have expected one or another senolytic therapy to beat lecanemab to the punch — and to do so with an approach that was more widely recognized to come out of work on longevity therapeutics.
Indeed, senolytic startups sprung up like weeds and wildflowers soon after Mayo Clinic researcher James Kirkland first discovered senolytic drugs — and the first out of the gate was UNITY Biotechnology. With a compelling-looking proof-of-concept senolytic therapy in an animal model of osteoarthritis; senescence research powerhouse (and SENS Research Foundation Research Advisory Board member) Dr. Judith Campisi and others as scientific cofounders; and standout biotech serial entrepreneur Nathaniel (Ned) David organizing and steering the company, its launch was an exciting milestone in our race toward a future free of degenerative aging.
Many people in the prolongevist community followed UNITY’s launch and progress with intense excitement. Upon learning of Dr. Campisi’s groundbreaking study showing that the different senolytic therapies prevented and partially reversed atherosclerosis in mouse models of the disease and that UNITY had secured a whopping US$116 million in Series B funding and was organizing human clinical trials of a novel senolytic, I myself dashed off an email that included the salient line “Bless you, tiny woman ☺.”
UNITY followed up that funding announcement with the release of a foundational animal senolytic study by UNITY-affiliated researchers. The study showed that ablating senescent cells in the knee with the novel senolytic drug UBX0101 blunted the development of osteoarthritis, reduced pain, and boosted the recovery of articular cartilage development in a mouse model of ACL injury. It was this experimental study that UNITY would take forward into its first clinical trial.
And the story became even more exciting when UNITY announced the results of its Phase I clinical trial of UBX0101 for osteoarthritis. The purpose of Phase I trials is just to test the waters, figuring out if a potential therapy is blatantly unsafe and what the ballpark of an appropriate dose would be, so companies speed things along and conserve cash by only enrolling enough people and running the trial long enough to answer those questions rather than the much larger numbers and longer timelines necessary to see whether the therapy works as intended.
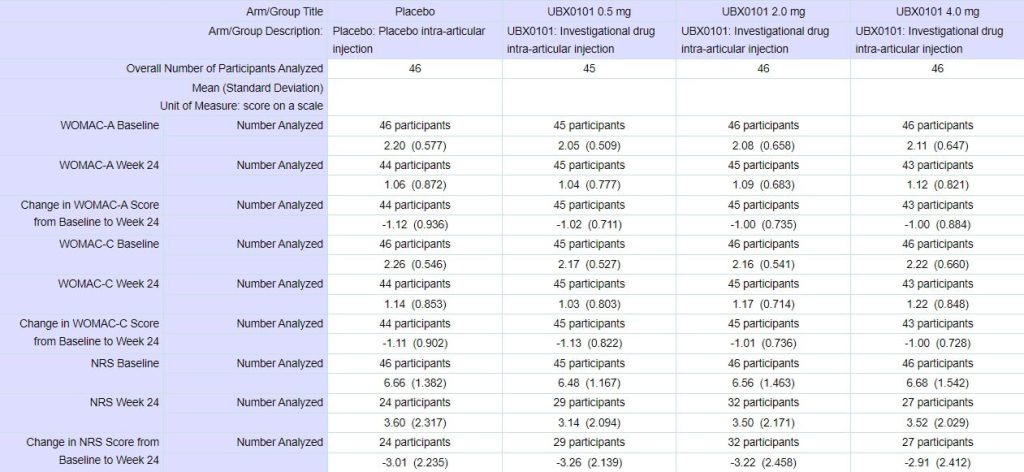
None the less, the UNITY scientists did test patients for pain indicators, and the results were tantalizing. Although preliminary — and although there was oddly inconsistent lack of effect at the midrange dose of 0.4 g — it appeared as though UBX0101 was indeed substantially reducing the level of pain suffered by people with osteoarthritis (average age 62; 48 participants), and that the higher the dose, the more effective it was.

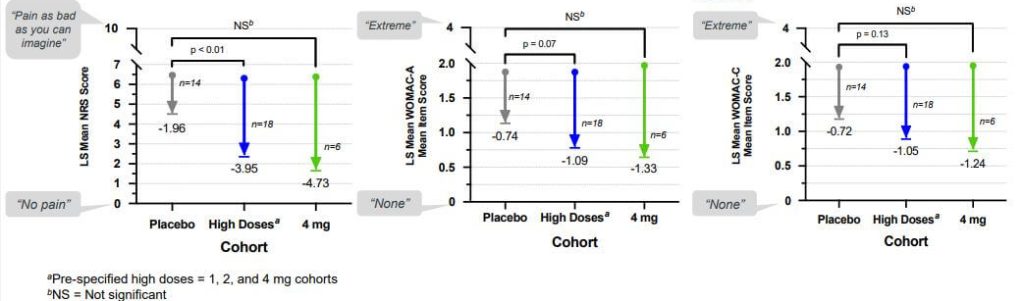
Importantly, the patients who got UBX0101 were still reporting lower pain scores than those who got placebo 12 weeks after they had been given a single dose of the drug. That result was consistent with the effect of a senolytic: unlike a mere painkiller which would merely damp down the production of inflammatory factors for as long as the drug was present in the knee, destroying senescent cells would demolish the cellular factories that produce inflammatory SASP factors in the first place. It would be like the difference between treating an infected injury with antibiotics to eliminate the invading microbes and just popping ibuprofen to tamp down a fever.
So investors and company scientists went into the Phase II trial with bated breath. This trial signed up more people (183 participants) and divided them into fewer dosing groups, including one group randomized to receive an injection of 4 mg of UBX0101 — double the highest dose used in the Phase I trial.
To the surprise of many, the trial was a complete flop. At the 12-week primary outcome date, patients getting UBX0101 did no better than those getting a saline placebo injection, no matter what dose of the drug they got. Regardless of how you squinted at the graph, it was a fail, and while the company said it would collect and share the results of the 24-week extension study and report it at a scientific meeting since doing so would require “immaterial costs to complete,” it was already clear that UBX0101 had flopped.

Some Explanations that Don’t Work
What went wrong? The company noted that there was an unusually powerful placebo effect in the trial, and argued that this anomaly might have obscured any effect of the senolytic. It’s true that the subjects who had been given placebo injections experienced much more pain relief than is typically gained from painkiller tablets, as seen in the Figure below, but this is not unique to this trial: it’s a known feature of injectable placebos that they give an especially strong placebo effect. This isn’t unique to injectables: the general rule is that the more dramatic the form and delivery of a placebo intervention is, the more effective it will be. Trials of sham surgical procedures (in which surgeons make an incision and then stitch it up without performing any active intervention) can give relief a remarkable 74% of the time — and about half of trials find that the relief is equal to that of the actual surgery. That compares to a 20-30% placebo response in most trials. People get more relief from an opioid or an antianxiety drug when they see a nurse injecting it into their IV drip than they do when an unseen automated system drips it invisibly into the line. And they also get more of an effect when the color of a placebo pill aligns with their associations of that color with its supposed effect.
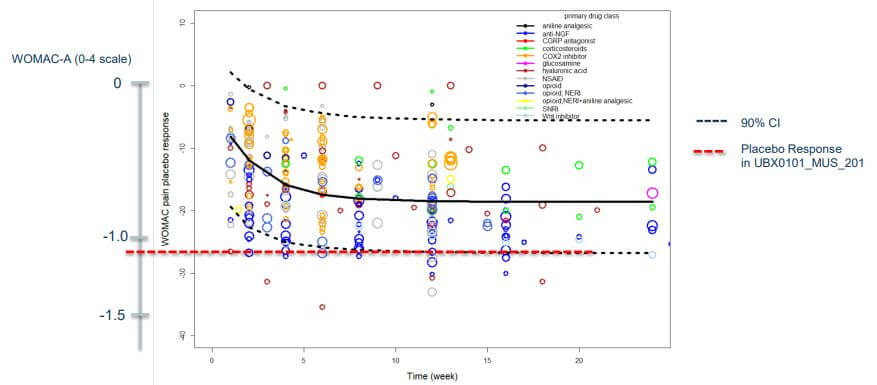
However, that doesn’t seem like an adequate explanation for the failure. For one thing, the same potent placebo effect of a saline injection ought to have been present in the Phase I trial just as it was in Phase II. Perhaps it might have been stronger in the Phase II trial because people enrolling in it would know about the very positive-looking results of Phase I; if UNITY management wanted to make a convincing case on this, they should have graphed out the placebo effect in the Phase I trial along with the Phase II.
But more importantly, one of the things that had made the Phase I results look so convincing was the apparent dose-response effect: the higher the dose (“and therefore,” hopeful prolongevists would mentally insert, “the greater the number of senescent cells destroyed”), the greater the benefit. But no matter how you squint at the results of the Phase II trial or at what angle you hold the graph, there’s just no hint of that happening Phase II.
Others suggested that the trial may not have lasted long enough for UBX0101 to show its stuff, recalling another potential disease-modifying therapy that had failed in Phase II but had just started showing hints of a benefit when the trial wrapped up at 24 weeks and also a cell therapy that had taken months to show its stuff. But in both those cases — whether delivering new cells into the knee to produce new cartilage or using a drug to try to stimulate the surviving cells to do the same — you’d expect it to take some time for the treatments to produce enough new collagen to result in actual pain relief, especially the tissue would still be inflamed and being ground down by daily use. By contrast, if a senolytic drug works, it executes its targets very quickly — and because senescent cells are little factories of inflammatory factors, pain relief should have followed right behind.
Also, there was something a bit puzzling about the time course in the original Phase I: although there seemed at first glance o be a rapid dose-responsive effect in the first few weeks after the injection, if you look at the graph, it actually looks like the lower doses of the drug were not only no better than the placebo by the end of twelve weeks, but had possibly left recipients in more pain than people getting saline! That doesn’t leave much room for thinking that the Phase II results might have delivered potent pain and functional relief if the investigators had just left the trial running a little bit longer. Plus, to remind you, UNITY included one dose of UBX0101 in the Phase II trial that was even higher than the highest dose they had tested in Phase I.
And there’s a third reason to doubt that the Phase II trial would have panned out if the investigators had allowed the trial to run a bit longer: the investigators actually did leave the trial running longer! Although the company announced the trial’s failure at week 12 (and more or less confirmed that they had given up on UBX0101 for osteoporosis in an investors’ teleconference soon afterward), they did continue observing the participants for a full 24 weeks as intended. Unfortunately, but there was nothin’ to see there either, folks.
Aging Ruins Everything
We may never know the exact reason for the split decision between the UBX0101 Phase I and II trials. But a close examination of the original experiment set that sent UNITY chasing osteoarthritis as the indication for their first clinical trial, in combination with the results of several much more recent studies, shows us that in choosing osteoarthritis as their first disease of aging to target, UNITY leadership failed to fully take on board how terrible degenerative aging really is.
UNITY-affiliated scientists performed most of their original animal studies in an animal model of ACL injury — specifically, ACL injury in young mice. Mice and people who have suffered one of these are at high risk of later osteoarthritis, so this is not a crazy way to test potential therapies targeting the disease. But certainly, a young animal (mouse or human) who develops osteoarthritis after suffering an ACL trauma does not have the same problem as an old animal that has developed the disease after accumulating intrinsic cellular and molecular aging damage combined with the low-level “wear and tear” that a joint suffers during ordinary use over a lifetime.
ACL injuries begin focally and away from the cartilage instead of being diffused throughout the joint (cartilage, ligament, and bone). And when a young athlete (or mouse) suffers an ACL, his or her cartilage, bone, AC ligament, and entire body are still young and functional, including significant numbers of surviving nonsenescent cartilage-producing cells. By contrast, every structure in the tissue undergoes degenerative changes in the osteoarthritis of aging — and it happens in the context of an entire body that is also aging, leading to circulating factors that inhibit the regenerative potential of the tissue.
Plus, an ACL injury is a highly focused insult to one part of the joint, resulting in different effects on the biomechanics of the joint than does the more widespread damage that accumulates in a joint with aging. Thus the two problems result in different kinds of secondary stress being placed on the joint as the organism attempts to hobble along with it.
This contrast was already apparent if you read the full published paper describing the UNITY-affiliated scientists’ animal experiments and gave all parts of it equal weight. Most of the report was focused on studies on young mice given an ACL and then treated with UBX0101 or activating the senolytic “gene therapy” p16-3MR. Senolytic-treated animals suffered less than a third as much erosion of their articular cartilage and far less abnormal calcification and loss of essential structural components of the tissue, and treated animals enjoyed a boost in the expression of genes involved in the production of the structural components collagen and chondroitin sulfate, suggesting that they were even regrowing new joint tissue.
These exciting results were the focus of the scientific paper’s abstract and the basis for the eventual clinical trial. And if we had any doubts that other scientists could replicate them, the results were confirmed independently in March of this year, with another group showing that giving rats injections of an entirely different senolytic after they had suffered an ACL not only destroyed senescent cells in the cartilage, but substantially blunted the development of osteoarthritic damage in the joint.
But the results were quite different when the UNITY-affiliated scientists tried the same thing in mice that were in a stage of aging similar to a current human 60-year-old. Even before being hit with an ACL injury, the older animals’ cartilage was already thin and lacking much of its normal structural components — and after the injury, it got even worse. And pre-existing aging also altered the distribution of senescent cells in the old mice’s cartilage after the ACL insult. Some of this was presumably because senescent cells would already have crept into the tissue across the lifetime. Some of it might have been because the same injury can cause a different pattern of damage in a joint that has already degenerated with age. And some of it might have been because of the weaker ability of the aged immune system to patrol and eliminate senescent cells as they erupted.
Treating the older animals with UBX0101 or the senolytic “gene therapy” INK-ATTAC still eliminated large numbers of senescent cells. But unlike in the young animals, it had minimal effect on the structural components of the cartilage, no effect at all on the degenerated bone surface, and either a small effect or none on the overall structure of the joint. And there was no corresponding jump in regenerative gene expression after senolytic treatment.
Of course, what’s most relevant to most aging people — including the people in the UNITY Phase II trial — is not the use of senolytics to prevent a recent ACL injury from degenerating into osteoarthritis, but to delay or treat osteoarthritis from aging and chronic injury alone. And this was the focus of two new studies that we’ll dig into now.
Tell Me About Your Pain
In the first animal study, researchers from Korea University tested the effect of senolytics in the osteoarthritis of aging mice. Mice lived without any intervention until they were 21-22 months old — similar to 75-year-old humans — and then scientists examined their joints after treatment with either a drug-free control injection or one of two well-studied senolytic drugs: navitoclax (also known as ABT-263) or the combination of dasatinib plus quercetin (D+Q). Prior to treatment, these mice had clear evidence of age-related cartilage degeneration and joints infested with senescent cells. Senolytic treatment rained destruction down on the senescent cells in the joint, and by extinguishing the senescent fuel, it cleared the atmosphere of the smoke of SASP.
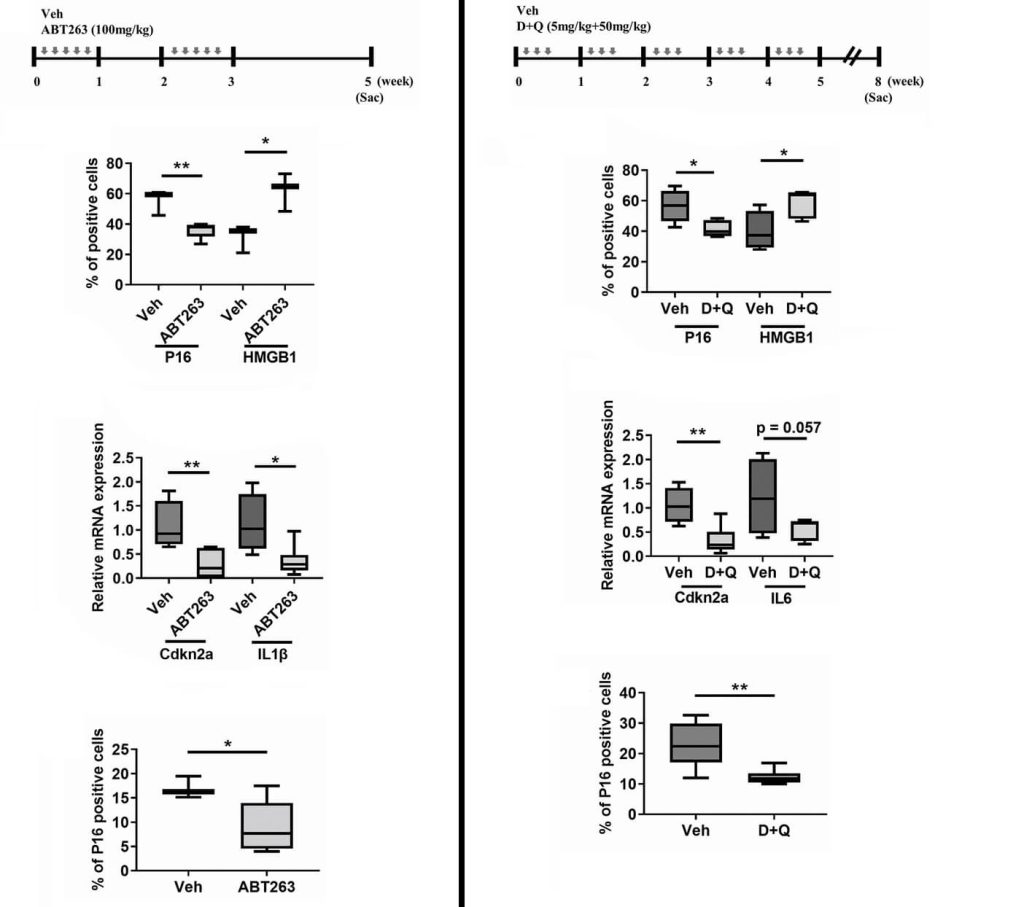
Senolytic treatment also gave the aging animals relief from joint pain. But despite that, neither senolytic did anything to improve the integrity of the cartilage, reverse the abnormal bony projections, or normalize the inflammation of the synovium (the thin membrane between the cartilage and the tendon).

The researchers went on to dig into the reasons why senolytic treatment relieved pain in these animals, showing that prior to treatment, the osteoporotic joint was stimulating the growth of pain-sensing neurons into the joints by stimulating the production of nerve growth factors even as they inhibited the growth of new blood vessels by reducing expression of their growth factors. (While the cartilage in joints lacks a blood supply (which is one reason the cartilage doesn’t regenerate), it does receive nourishment from fluid produced by the synovium. But the synovium is inflamed and dysfunctional in osteoarthritis, and being denied a blood supply would further impair its life-giving support to the cells in the cartilage.
Senolytic treatment put a stop to all of those problems and alleviated the animals’ pain. The researchers didn’t connect the dots, but a plausible explanation is that factors in the SASP were both triggering the tissue to produce nerve growth factors and blunting the production of chemical messengers needed for blood growth — and having recruited pain-sensing nerves into the tissue, inflammatory factors in the SASP irritated those same nerves. Senolytic treatment put the kibosh on all of these effects by eradicating senescent cells and thereby snuffing the SASP out.
This study doesn’t explain as much as we might want about what happened in the UNITY Phase II trial. The endpoint of the trial was to show that UBX0101 would relieve pain and improve function (which in osteoarthritis is often limited by pain), so if killing senescent cells in true aging-driven osteoarthritis can alleviate pain without improving the degenerated joint, you would expect the UNITY Phase II trial to have succeeded. And yet, of course, it didn’t.
But the new paper does emphasize that UNITY would have been well-served to have taken the additional step of testing UBX0101 in a model that better reflects the suffering patients they’re trying to help, focusing on testing drugs intended to treat the osteoarthritis of aging in people in old mice with the same problem instead of leaning so heavily on results gleaned from injured young mice.
Did Even the Early Bird Miss the Worm (of Aging)?
You might think that the problem with the Korean study was that the mice only began getting senolytics when it was too late: if the scientists had instead begun administering senolytics earlier in life, before so much recalcitrant structural damage had set in, it might have prevented the joint from getting so bad in the first place. That’s an attractive idea: UNITY had focused on the inflammatory signaling molecules in the SASP, but the SASP also contains enzymes that degrade the ECM and (as we saw in the Korean scientists’ work) inhibit the growth of blood vessels needed for the synovial membrane to nourish the joint. So rooting out senescent cells early might have put a stop to the SASP before it had the chance to cause late-stage damage that senolytics would not be able to reverse.
So another study set out to test the ability of senolytics to prevent degenerative skeletal diseases in mice (including degenerative disc disease, osteoporosis, and osteoarthritis), starting either when they were young adults (6 months old), middle-aged (14 months), or in the early aged period as we encounter it today (18 months). The best outcome of all of these studies was that — in line with previous studies — the senolytic combination D+Q significantly reduced the loss of bone mass and other aspects of osteoporosis in the mice, no matter the age at which they started treatment. (We’ll note, however, that in a separate study, a “gene therapy” senolytic approach failed to rejuvenate bone when started at an even later age).
The results in degenerative disc disease were somewhat as we speculated they would be for using senolytics against osteoarthritis at different time points above. Delaying senolytic treatment until the mice were in the equivalent of their 50s or 60s in today’s biological ages failed to improve the animals’ disc degeneration. But starting senolytic treatment when the mice were in young adulthood or mouse middle age substantially preserved the discs’ integrity.
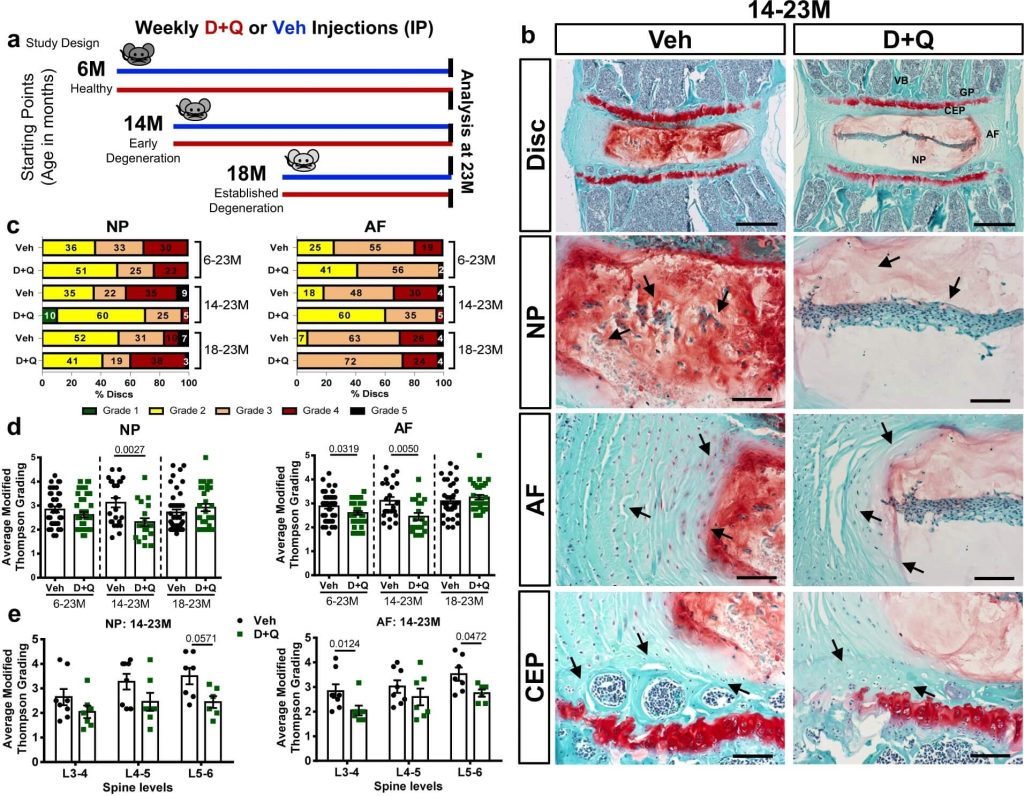
However, the effects in osteoarthritis were disappointing — but came with a curve ball that makes it impossible to draw firm conclusions from the results. On its face, D+Q treatment was a bust, having no effect on joint degeneration no matter what age the animals started treatment. But there’s an immediately apparent if puzzling explanation for why the drugs couldn’t have succeeded regardless of the merits of targeting senescent cells in osteoarthritis: at least in this study, D+Q failed to kill any senescent cells in the joint!
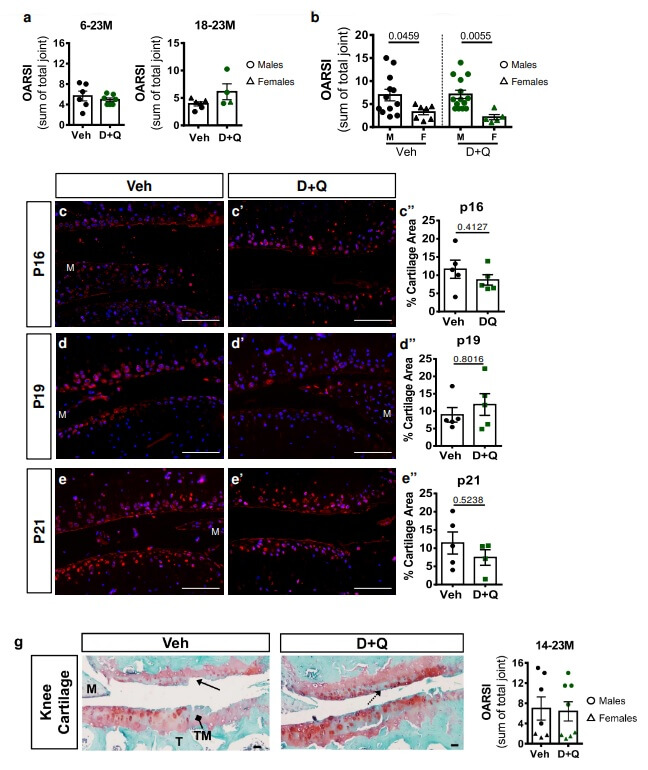
Why would D+Q fail to kill senescent cells in the knee joints of the mice in this study when they successfully destroyed them in the skeleton and in the discs or the spine — or when D+Q eliminated senescent cells in the independent study we reviewed earlier on? The researchers themselves don’t explore the issue in their paper, and the two studies used the same doses of D+Q from the same source.
There was a difference in the solvents the two studies used to dissolve the D+Q. Neither group of scientists used the same solvent cocktail used by James Kirkland and his team, who launched the field of senolytic drug therapy with the discovery that D+Q selectively obliterates senescent cells: the Korean study used a related solvent, and the study we’re discussing now used a completely different one. By the time the drugs reached the spine bones and discs and the joints, D+Q would be carried in proteins in the plasma and dissolved in the plasma itself (respectively), regardless of how the scientists first administered them. But might the carrier in the Korean study have allowed the mice to absorb more of the drugs, or absorb them at a different rate, such that the concentration of D+Q that reached the various tissues was high enough in the spinal bone and discs to kill senescent cells, but not high enough to be effective in the joint? It’s speculation all, unfortunately.
Using the Right Tool — and Using the Tool Rightly
The mouse study we just discussed, in which D+Q failed to do its minimal job of destroying senescent cells in the joint, prompts the question of whether UNITY researchers did enough to optimize either the choice of senolytic drug or the dose and means of delivery to get the best performance out of their weapon.
What if UBX0101 didn’t actually kill senescent cells in human joint tissue? You would think we would know the answer to this fundamental question, but we actually don’t. While the UNITY-affiliated scientists’ original paper focused mostly on mouse studies, they also showed that UBX0101 selectively killed senescent cells extracted from whole knee tissue taken from patients with osteoarthritis undergoing total knee replacement surgery. However, the killing rate was low: merely 20% of senescent cells died, versus a net 5% of nonsenescent cells. So to clear out enough senescent cells to ensure that patients would feel the difference might have required either a better senolytic or multiple administrations of the drug instead of the one-time treatment used in the trial — and if they had chosen the latter path, it might come at the cost of unacceptable collateral damage to the dwindling supply of viable cartilage-producing cells (chondrocytes).
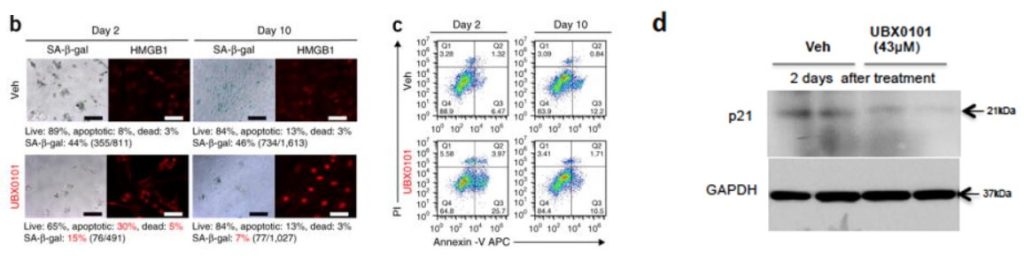
On top of that, the cells in this preliminary test were extracted from the tissue and then grown in culture rather than embedded in the complex matrix of the original joint, where the drug might not have reached them at adequate concentration. And in the rodent studies, UBX0101 was rapidly cleared out of the joints after injection, with concentrations falling below the concentration expected to kill half of the cells after just 90 minutes.
If you put all of these apparent limitations together —a weak senolytic that may not have even reached all the cells in the joint before being quickly cleared out of the tissue — it’s reasonable to wonder if UBX0101 might have failed because it simply could not kill enough cells to help patients.
Unfortunately, we never got a good readout on UBX0101’s effectiveness as a real-world senolytic from the human trial. Testing the numbers of senescent cells killed in the treated patients’ joints would have required surgeons to remove the patients’ cartilage afterward — which would have then required them to get knee replacements, which would rather have defeated the purpose! But the result was that we’ve never had a measurement of UBX0101’s senolytic action in the tissue of actual human patients. The investigators did try to extract some of the fluid bathing the joint to look for markers of SASP but were never able to get fluid that reliably reflected the conditions inside the joint, so we didn’t even get that indirect evidence.
And even if we knew for sure that UBX0101 was executing large numbers of senescent cells when injected locally in the joint, that might not have been the best way to administer the drug. Scientists and doctors chose to inject the drug directly inside the knee joint in both the animal experiments and the clinical trials for several reasons. It would maximize the concentration of the drug directly in the knee cartilage, yet cut down on the total amount of medication needed (since you only need enough for that one small area of tissue) and minimize exposure of the rest of the body to the drug. That last point would reduce the risk of side-effects in other tissues, which was attractive in itself and would also have scored them points with regulators and investors.
However, we now know that the benefits of senolytic treatment in a given tissue aren’t just the result of killing senescent cells in that tissue, but of broader changes that happen across the entire organism when senescent cells are killed elsewhere. For example, destroying senescent cells exclusively in bone tissue prevented bone loss in mice’s spines, but not their femurs — and while it improved bone formation, it didn’t have any effect on osteoclasts (the main bone-degrading cells) or prevent the diversion of precursors of bone-forming cells into becoming fat cells (adipocytes) instead. But when scientists treated mice with senolytic drugs that killed senescent cells everywhere in the body, it not only improved bone formation but also cut down on the emergence of osteoclasts and misplaced adipocytes.
Similarly, exposing young mice to the blood of old animals causes their organs to sprout senescent cells and age prematurely, and much of this “pro-aging” effect can be prevented by treating the old animal with senolytics before taking its blood to give to the young animal. This means that factors produced by senescent cells and carried in the blood can age organs even if those organs don’t begin with any senescent cells of their own.
So despite the potential advantages of concentrating UBX0101 in the tissue they wanted to affect, it’s possible that a more typical whole-system senolytic treatment would have brought more pain relief and structural improvements to the participants in their trial.
When More Damage Has Accrued, Repair More Damage
Where do we go from here? Certainly, we could give up on using senolytic therapies to prevent and reverse osteoarthritis entirely, but that would be premature. All of the animal studies we reviewed in this article and many more agree that senescent cells build up to alarming levels in both young injured cartilage and in cartilage ravaged by aging processes alone. We can’t afford to ignore this damage, and the effects of senolytic therapy in the cartilage of young, injured animals and in other tissues in uninjured aging animals are promising enough that we should continue looking to find the right way to use these remarkable rejuvenation biotechnologies to bring relief to aging, aching joints. When you step back, the results of data from each of these studies give us some hints of where they would best fit into a future osteoarthritis rejuvenation protocol.
First, as we discussed a moment ago, there are enough questions about UBX0101’s actual senolytic effectiveness and about the wisdom of focusing all the senolytic firepower exclusively in the joint to merit experimenting with different senolytic therapies and with using a whole-body approach to make the best use of the senolytics themselves. Once they are mature, this could include the immune-based senolytic therapies our scientists are developing at SENS Research Foundation.
Second, in the original UNITY-affiliated animal study, the investigators noted that when they treated young injured animals with UBX0101, their chondrocytes ramped up the activity of genes involved in producing new collagen — but the old mice’s joints failed to respond the same way. Equally importantly, senolytic treatment did not trigger a jump in the expression of Sox9 (a critical gene for the production of new chondrocytes) in either young or old mice. So it seems likely that one reason for the failure of UBX0101 to regenerate the joint in the old animals was some combination of having few surviving chondrocytes and that those chondrocytes were senescent or otherwise dysfunctional.
In fact, we already know that those exact problems occur in aging human joints. Autologous chondrocyte implantation is a form of cell therapy used to repair isolated cartilage damage and defects; an older procedure called osteochondral grafting can be used for combined bone and cartilage injury, and a newer technique called autologous matrix–enhanced chondral transplantation (AMECT) is being used experimentally for damage to the hip socket cartilage. In these treatments, surgeons harvest healthy cartilage from a non-weight-bearing joint elsewhere, grow out large numbers of chondrocytes in the lab, and then implant them back into the tissue (sometimes with supporting extracellular matrix or growth factors), where they can help regrow the tissue and hold off osteoarthritis and a joint replacement. Promisingly, there’s evidence that it can even delay a knee replacement in younger patients who have already developed osteoarthritis after an injury.
But guidelines generally advise against getting these procedures once one gets into one’s late 40s and 50s — and if such patients opt to undergo these treatments anyway, then insurance companies usually refuse to pay for them because the outcomes aren’t as good and older people often still wind up getting the joint replacement they were trying to avoid.
One of the critical reasons why these procedures don’t work as well in older subjects is that an older person’s chondrocytes grow more sluggishly in culture and don’t produce as much collagen — just as was seen in the older animals in the UNITY animal study, despite senolytic treatment. Scientists are working to solve this problem: for example, one group has found that they can boost the rate of chondrocyte replication in culture much of the way back to the youthful norm by using a custom cocktail as a growth medium instead of the usual calf serum.
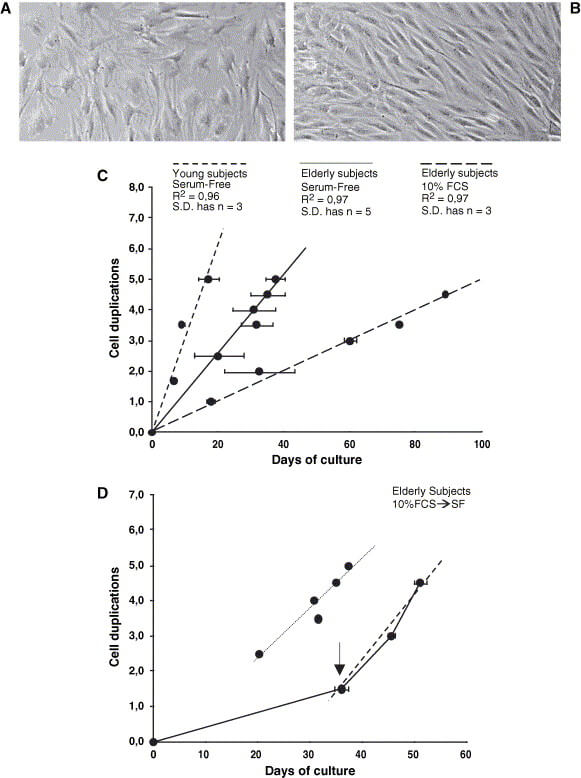
Other scientists have found that growing chondrocytes harvested from older patients on standard hard plastic surfaces prevents them from developing a normal, youthfully rounded shape, and that growing them in a suspension culture instead both normalizes their shape and allows them to proliferate more youthfully. And still other scientists are working on new ways to deliver and support the cells once they get where they’re going.
While the current cutting edge is to these tricks to make old cells work better, the next step is to use cellular reprogramming to take a patient’s own cells and transform them into fresh, new chondrocytes in much larger numbers than is possible today. Since this would overcome some of the key limits of senolytic therapy seen in the animal experiments, you might expect a “remove-and-replace” strategy of ablating senescent cells and then delivering fresh new cells into a more hospitable post-senolysis environment to work better than either therapy alone.
And vice-versa, senolytic treatment did boost collagen and chondroitin sulfate production in the young animals in the UNITY studies, which might be important to the successful use of reprogrammed “young” cells, since a study using radioactive isotopes to carbon-date knee tissue revealed that no new collagen is laid down in the joints during adult life in either healthy or diseased joint tissue. Alternatively, scientists could treat therapeutic cells to give them a temporary extra boost in expression of the pro-regeneration gene Lin28 when surgeons first implant them: even without new cells or senolytic treatment, ramping up Lin28 expression has been shown to stimulate chondrocyte proliferation and protect against cartilage breakdown in a better mouse model of osteoarthritis than ACL injury.
And to fully rejuvenate the osteoarthritic joint, we may well need to develop additional rejuvenation biotechnologies to address other kinds of damage beyond cell loss and dysfunction and accumulation of senescent cells. In the UNITY animal studies, ACL injury and the development of osteoarthritis triggered genes that encode bone-resorbing genes and led to malformation of the bone tissue just below the cartilage. Senolytic “gene therapy” did nothing to reverse those changes, pointing to an opportunity to even further rejuvenate the joint through bone-repair therapies.
UNITY’s Future — and All of Ours
Fortunately, UNITY did not fold up its tents and end its push to fight to harness senolytic science against the diseases of aging. They are now advancing several programs using a new senolytic (UBX1325) with a different senolytic mechanism of action through both animal studies and clinical trials against several degenerative eye aging diseases. Excitingly, while UBX0101 failed in Phase II, UBX1325 has already passed that bar with flying colors as a potential therapy for diabetic macular edema (DME): 24 weeks after receiving just one shot of UBX1325, DME subjects regained about 8.5% of their vision loss, while subjects on the placebo lost a further 2% of their vision. And 59.4% of subjects who received UBX1325 didn’t require any additional treatment with the standard of care for DME (an antibody that inhibits the growth of blood vessels), whereas only 37.5% of subjects who got the placebo were able to get by without this rescue therapy. Results of 48 weeks of followup are expected next month (April of 2023).
Degenerative aging is a monster, and while a single silver bullet might have many benefits, keeping people young and healthy in body and brain will require us to remove and repair all the cellular and molecular damage of aging. We must learn what we can from the UNITY trial’s failure to push forward into a future of joints free of pain and movement with the freedom of our youth.