
SENSible Question: The Hallmarks of Aging has become an extremely widely-used framework for categorizing aging changes. For the purpose of having a shared language, would it make sense to simply adopt the Hallmarks in place of the seven strands of SENS, while still attacking the damage of aging using the SENS “damage-repair” heuristic?
That’s a great question. As of the end of September 2023, the original Hallmarks of Aging paper had been cited 12,801 times in the scientific literature according to Google Scholar, whereas the paper in which a version of the Seven Strands of SENS first appeared has “only” been cited 270 times, despite having been published a decade earlier and with equally prominent coauthors.
At the time that Dr. Aubrey de Grey and colleagues first proposed SENS, all the aging interventions scientists had successfully used in laboratory animals — Calorie restriction, mutations in the insulin/IGF-1 pathway, and (a few years later) the drug rapamycin — were single metabolic levers. Scientists had learned they could pull any one of these levers and slow down many aspects of aging at once, resulting in an extension of healthy lifespan.
A key innovation that Dr. de Grey introduced with SENS was the idea that instead of trying to tackle all of aging at once, aging could be delayed or even reversed using a piecemeal “divide-and-conquer” approach. The paper identified discrete categories of cellular and molecular aging damage whose particular members were similar enough that scientists could deploy common strategies to remove, repair, or replace the constituent lesions. And if that categorization system were comprehensive enough, then attacking all of its constituents individually would be enough to delay or even reverse the whole organism’s aging.
At the time, this strategic insight was novel and largely untested, and it attracted some pushback from otherwise right-minded biogerontologists: “de Grey fails to mention that none of these approaches has ever been shown to extend the lifespan of any organism, let alone humans.” As Dr. de Grey noted in response at the time, it was (and is) true that “no SENS intervention—in isolation—has ever been shown to extend any organism’s lifespan. I do not recall Henry Ford alerting potential customers that the components of a car—in isolation—remain obstinately stationary when burning petrol is poured on them”.
The Hallmarks paper arrived a few short years after that heated exchange, and now — a decade further on — the Hallmarks categorization system has become a scientific lingua franca for talking about different kinds of changes in the aging organism. And as scientists have gotten used to thinking and speaking about aging in this segmented way, it has become entirely mainstream to think about tackling aging by targeting individual Hallmarks rather than master regulatory switches for the process as a whole. With this important shift in scientific thinking achieved and with the Hallmarks being so widely used and understood, is it now time for the SENS Seven to “fade away into glorious obscurity” in favor of them?
The short answer is “no.” While the Hallmarks paper used rational criteria for identifying features of aging that are causally tied in some way to the rising risk of death and disease, those criteria are not strategically organized around the goal of identifying a suite of targets to bring aging under full medical control. Lacking an overarching strategic framework like the SENS “damage-repair” strategy, they tended to focus on “Hallmarks” in the literal sense of “characteristic features” rather than in the biomedically useful sense of “critical targets.” As a result, they wound up with a mixture of causes and effects of aging, and with things that are “causes” in different senses. And as we’ll see, the Hallmarks paper also misses other critical targets, while including others that are redundant to each other.
The Parting of the Ways
Both the Hallmarks and the SENS Seven begin from the consensus position that (as the Hallmarks paper put it) “The time-dependent accumulation of cellular damage is widely considered to be the general cause of aging (Gems and Partridge, 2013; Kirkwood, 2005; Vijg and Campisi, 2008). … Therefore, cancer and aging can be regarded as two different manifestations of the same underlying process—namely, the accumulation of cellular damage.” The Hallmarks paper then sets out its criteria for identifying potential Hallmarks: “Each hallmark should ideally fulfill the following criteria: (1) it should manifest during normal aging; (2) its experimental aggravation should accelerate aging; and (3) its experimental amelioration should retard the normal aging process and hence increase healthy lifespan.” However, they give themselves significant leeway on that last criterion:
The last criterion is the most difficult to achieve, even if restricted to just one aspect of aging. For this reason, not all of the hallmarks are fully supported yet by interventions that succeed in ameliorating aging. This caveat is tempered by the extensive interconnectedness between the aging hallmarks, implying that experimental amelioration of one particular hallmark may impinge on others.
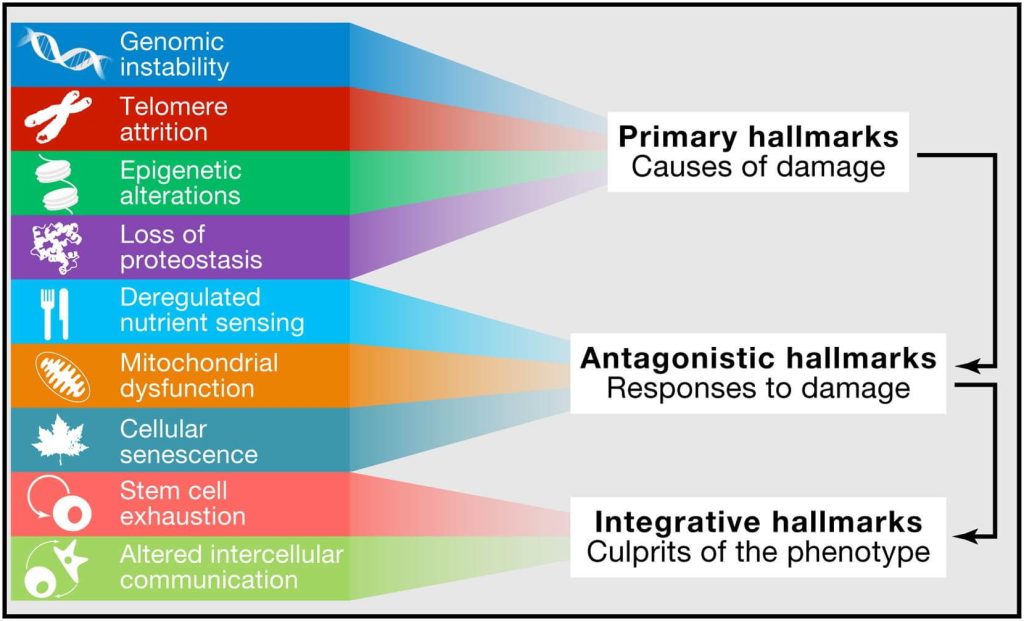
With those criteria in hand, the authors wound up with “nine hallmarks of aging [that] are grouped into three categories”: primary Hallmarks, compensatory (or antagonistic) Hallmarks, and integrative Hallmarks.
The paper defines primary Hallmarks as “primary causes of cellular damage” and elsewhere as “the physiological sources of aging-causing damage.” Take careful note of the phrasing, because this is the first and critical divergence between the Hallmarks and the SENS Seven. The authors did not develop the Hallmarks as a system to organize the cellular and molecular damage of aging itself, but the metabolic processes that contribute to the rate at which that damage accumulates.
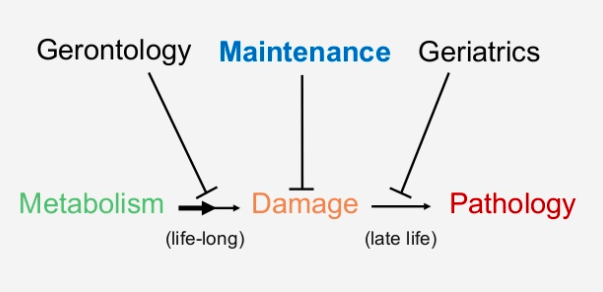
By contrast, the SENS Seven are precisely a systematic categorization of the damage of aging itself. This is because such damage is the exclusive target of the SENS “damage-repair” strategy, and the SENS Seven are organized around platform technologies that scientists can retool to attack multiple particular molecular lesions within each category.
This blog post isn’t the place to rehash the case, but Dr. de Grey’s key insight in developing the SENS heuristic was that leaving “the physiological sources of aging-causing damage” to operate without interference and instead attacking aging damage directly is a superior strategy for the medical conquest of aging. The damage-repair approach sidesteps competing risks tradeoffs; lowers the risk of side effects; and removes and repairs the cellular and molecular damage that an aging person has already accrued when he or she starts therapy rather than just slowing down the rate at which a person accumulates more aging damage going forward. And because it is amenable to iterative cycles of improvement, the SENS strategy opens the potential for longevity escape velocity once scientists have developed and deployed a sufficiently comprehensive first panel of rejuvenation biotechnologies. (Some of these points are the subject of a previous blog post, and we made the case for the SENS approach in detail with all the science you need to understand the overall plan in Ending Aging).
Another thing that’s problematic about focusing on the “sources of damage” rather than the damage itself as the basis for a catalog of modifiable age changes is that it’s not actually the sources of damage that change with age. You might imagine that our bodies are free of aging-damaging processes when we’re young and that a switch gets flipped somewhere in middle age, after which our bodies start wrecking themselves. For the most part, this isn’t true: to the extent that we can track it, our bodies produce most aging damage throughout our lives, and even the rate at which it’s generated doesn’t consistently rise with age. The burden of beta-amyloid plaque in the brain increases progressively until a person reaches the point of having substantial cognitive impairment, at which point it plateaus; some populations of mitochondria produce more free radicals later in life, but other populations appear to produce them at a constant rate — and production likely decreases to next to nothing in the small number of cells overtaken by mitochondria with large deletions.
What unambiguously changes progressively with age is the accumulation of cellular and molecular damage, not the sources of that damage. And again, it’s this damage — its accumulation, and its removal or repair — that is the sole focus of the SENS Seven, and not where the damage comes from.
An additional difference between the Hallmarks and the SENS Seven at this foundational level (and a limitation of the former) is that the original Hallmarks focus myopically on cellular damage, which ignores other kinds of aging damage that occur outside and beyond the cells — damage that is encompassed in the SENS schema.
We’ll circle back later in this post to go through the specific Hallmarks in each of these subcategories; for now, let’s look at the next subgroup of Hallmarks.
Heal Your Fracture and Cast Aside Your Crutches
The second subset of Hallmarks are “compensatory or antagonistic responses to the damage. These responses initially mitigate the damage, but eventually, if chronic or exacerbated, they become deleterious themselves.” This is one of the features of degenerative aging that make treating the particular “diseases of aging” so difficult. The body responds to cellular and molecular damage by attempting to counteract the effects of that damage, such as by sending in the immune system to remove damaged cells or molecules; or by pushing harder on the biochemical gas pedals to rev up a function that is declining due to damage to its cellular and molecular engine; or by turning down a process that is essential to normal function but that is generating too many problems because of such damaged cellular and molecular parts (like gearing down a faulty, pollution-spewing engine).
Such maladaptive responses to cellular and molecular damage are certainly characteristic features of aging, so they fit well into the original design plan for the Hallmarks. And we’ll come back later in this post to evaluate the specific aging changes that they assigned to this subcategory. But when we look down from the 30,000-foot level to consider the Hallmarks as a roadmap for tackling aging, what would we do about changes that happen in aging as a way to compensate for a rising burden of aging damage?
These “antagonistic” changes help a broken system hobble on — but they don’t solve the underlying problem for which they’re compensating — and eventually, such (mal)adaptive responses themselves cause problems with our health. So it’s harmful to leave them unchecked — but it’s also harmful to interfere with them directly, because doing so denies the body its best attempts to sustain normal function. It’s like pulling out the crutches from under a person hobbling along with a sprained ankle. If you get in the way of the body’s flailing attempts to tread water when it is at sea in an ocean of aging damage, you may drown a person in your attempt to save them.
The Janus-faced nature of such responses to aging damage is one of the dilemmas of the traditional geroscience “messing-with-metabolism” approach. In turn, it is one of the most clear-cut arguments for the SENS “damage-repair” strategy of exclusively targeting cellular and molecular damage itself, leaving metabolic compensations — and indeed, all of metabolism — to run its course uninhibited.
Instead of falling into a cycle of therapeutically pushing back against processes that are themselves forms of metabolic pushback, we should bypass metabolic adaptations entirely and target their causal drivers. Just as a fever breaks once the immune system clears a viral infection, so the body’s “compensatory or antagonistic responses to the damage” will become unnecessary and fade away once we have removed the cellular and molecular damage that necessitates them.
In contrasting the compensatory Hallmarks with the SENS approach, we’ve actually been creating a bit of a false dichotomy — or rather, we’re consciously putting forward a false dichotomy created by the Hallmarks schema, when in fact some of the compensatory Hallmarks either are or closely overlap with members of the SENS Seven. We can thus target such Hallmarks directly with SENS rejuvenation biotechnology. Again, we’ll come back to the particular age changes that the Hallmarks paper categorized as “compensatory” later on in this post.
Communication Breakdown
The last subgroup of Hallmarks are the integrative Hallmarks, which the paper says “arise when the accumulated damage caused by the primary and antagonistic hallmarks cannot be compensated by tissue homeostatic mechanisms … and are ultimately responsible for the functional decline associated with aging.” Note again that the overarching conceptual framework of the Hallmarks is in lockstep with SENS: aging is driven by cellular and molecular aging damage, and other age changes are downstream effects of that damage. But it doesn’t seem justified to say that the two Hallmarks slotted into this subgroup — stem cell exhaustion and altered intercellular communication (that is, changes in signaling between cells) — even belong in the same category, let alone that they “are ultimately responsible for the functional decline associated with aging” in a way that other Hallmarks are not.
Altered intercellular communications of one kind or another are rife in aging, but it’s odd to say that such changes arise when other kinds of aging damage “cannot be compensated by tissue homeostatic mechanisms,” since most changes in intercellular communications are tissue homeostatic mechanisms! That is, in most cases, the aging body shifts its signaling in characteristic ways in an attempt to re-establish normal function by counteracting, compensating for, or stimulating the loss or aberrant function of damaged cellular and molecular machinery. In fact, altered intercellular communications are a clearer example of a compensatory Hallmark than any of the things the system classifies as compensatory Hallmarks!
For example, atherosclerosis begins when LDL particles become trapped in the artery wall. In response, the artery produces signaling molecules that cause the cells lining the artery to put out “Help Wanted” signs that recruit immune cells called macrophages into the artery to remove the offending particles. This immune clearance strategy works well in the short term, but over time the burden of lipoproteins outstrips these immune cells’ ability to process them (especially if the level of circulating LDL is high). To compensate, failing macrophages send out inflammatory signals that attract even more immune cells into the vessel, accelerating the disease and eventually triggering heart attacks and strokes.
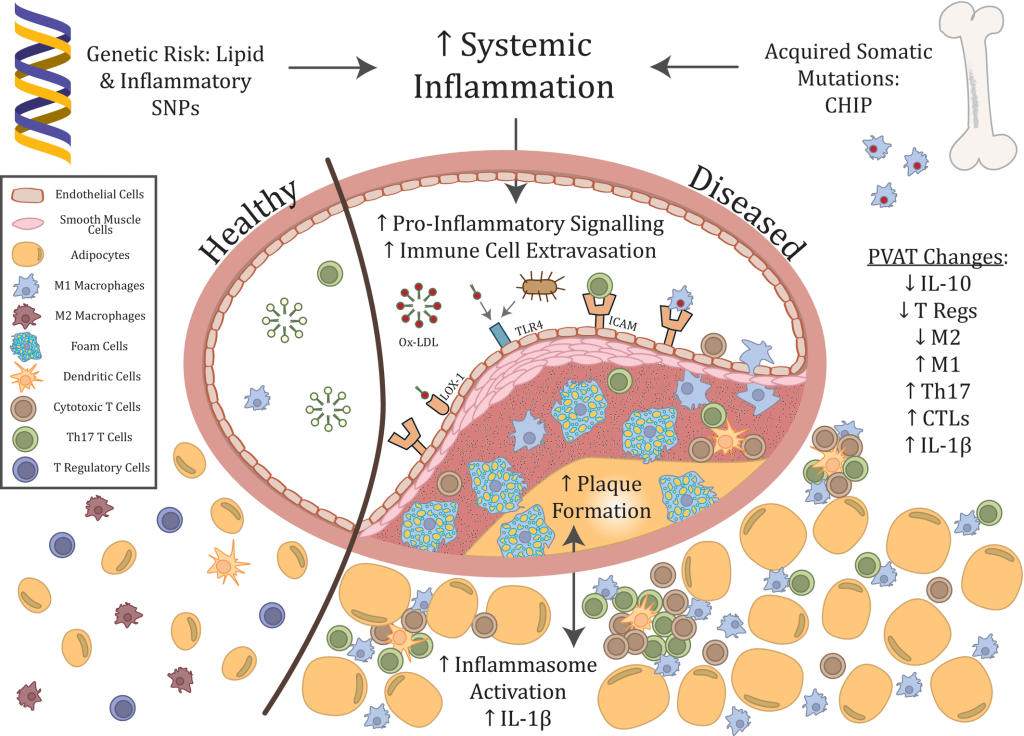
The body produces all of these inflammatory signals to mobilize the immune system to clear damage out of the artery, and both the immune cells and the inflammation are protective in the short term. But the longer they fight a losing battle, the more they devolve into agents of destruction, like Kurtz and his men after too many years in the jungles of Apocalypse Now. So what to do about them? Administering drugs that block the signals that recruit immune cells to the artery would allow damaged lipoproteins to injure the arteries even more directly, and forcibly suppressing the inflammatory reaction would undermine the role of inflammation in the immune system and other functions.
This dilemma is illustrated by a major clinical trial in which doctors treated people who had previously suffered a heart attack with an antibody called canakinumab that directly and indirectly tamps down on inflammatory messenger-molecules. In one sense, the trial was a success: the high-risk subjects who received the antibody had a 15% reduction in future heart attacks compared to those who received placebo shots. Yet despite that seemingly life-saving benefit, canakinumab-treated subjects didn’t actually live any longer — because in shutting down the immune system’s inflammatory response, the antibody also increased the risk of deadly infections.
There’s a very similar story behind inflammation in the brain afflicted with neurodegenerative aging of the Alzheimer type (AD) and the activation of brain-resident immune cells called microglia. Microglia are like the brain’s macrophages: they gobble up particulate matter, cellular debris, and other harmful materials in the brain — including, importantly, beta-amyloid, the extracellular aggregate most closely linked with AD. This happens because beta-amyloid activates the complement system, which flips the switch on microglial surface receptors that push them from being agreeable neuronal housekeepers into beast mode. But with a rising burden of beta-amyloid and unresolved injury to the brain, microglia and the complement system eventually become toxic, killing neurons and warping and shriveling the connections between them.
Other common derangements of intercellular communication that begin as adaptations to cellular and molecular aging damage include when the body responds to insulin resistance by producing ever more insulin, creating a vicious cycle that culminates in type 2 diabetes; and the hormonal signals behind hot flashes, which result from the pituitary gland’s increasingly shrill attempts to stimulate the release of a faltering supply of eggs.
In addition to regulated shifts that the body initially made in order to maintain normal function as the burden of damage rises, aging also distorts signaling networks because of the direct effects of aging damage to cells and biomolecules. Examples include the hodgepodge of signaling factors, growth factors, and enzymes (SASP) spewed out of senescent cells and the systemic oxidative stress indirectly exported from cells overtaken by mitochondria bearing large DNA deletions (which distort the use of free radicals as a signaling system).
In a recent update of the Hallmarks, the authors of the original Hallmarks paper wrote “that the final hallmark that we listed in 2013, altered intercellular communication, was too vast, requiring a separate discussion of chronic inflammation and age-associated dysbiosis.” From the SENS perspective, however, even the original creation of a separate Hallmark for altered intercellular communications was redundant at best and a counterproductive distraction at worst — let alone splitting off a new Hallmark by drawing an arbitrary line between age-related chronic inflammation and other kinds of signaling changes with age.
Again: Dr. de Grey, the authors of the Hallmarks paper, and the majority of aging and longevity scientists (even those who use language that suggests otherwise) agree that altered intercellular communications in aging are downstream effects of primary aging damage. If we are to set up a structure to classify age changes that doubles as a battle plan for tackling aging, we should use a version of Occam’s Razor to slice through these secondary changes and cleave as close as possible to the “bones” of degenerative aging: the cellular and molecular damage that drive such changes in the first place.
To develop powerful and iterable longevity therapeutics and minimize side effects, changes in intercellular signaling with age should not be a target for intervention. What we said earlier about compensatory Hallmarks applies more broadly here: the proper target for longevity therapies is the damage that causes these signaling changes, not the signals themselves.
In this first of two posts, we’ve looked at the thinking behind the structural framework into which the Hallmarks are organized. In next month’s post, we’ll come back and look at each of the Hallmarks individually and see how well they line up with the SENS Seven — and when they don’t line up, or when they overlap with more than one of each other, we’ll ask why the two systems differ, and what might be missing or superfluous in either system.



