Short summary: Another clinical trial has now proven that a second AmyloSENS rejuvenation biotechnology that clears beta-amyloid aggregates from the brain also slows down the slide into dementia in people in the early stages of Alzheimer’s disease. Some in the prolongevist community are downplaying the significance of these recent successes. A fuller understanding of the context reveals how remarkably successful these therapies are, why so many previous similar-sounding trials with other antibody treatments have failed — and what we need to do next to not only slow down, but arrest, prevent, and reverse the course of this form of neurodegenerative aging.
The news is fantastic.
For all of human history, there was no known therapy that had any measurable impact on the slow, terrible slide into neurodegenerative aging of the Alzheimer’s type (AD). People would spend decades appearing outwardly to be cognitively normal, even as critical cellular and molecular damage accumulated insidiously in their brains. Then cognitive impairment would set in, beginning with relatively subtle deficits: people would begin to have trouble making reasonable plans to achieve routine tasks in life, start forgetting recent events, or get confused too often in going about their daily activities. Then the memory deficits would get worse, their decisions would become less rational, they would have an increasingly difficult time putting their thoughts and feelings into words, and they would have a harder time handling normal financial decisions or handling the math of daily life. Their personalities would sometimes change in saddening or even frightening ways.
Eventually, all of these deficits would become so disabling that they couldn’t be left to live their lives independently, and they would need help even with progressively more basic activities of daily life. The neurodegenerative process could even rob them of their ability to swallow and to control their bladders or bowels. They would often not know the families that stood by helplessly trying to help them, looking intently into their eyes, clinging desperately to the brief flashes of humor, insight, or recognition before darkness would set in again.
The downward slide could be faster or slower, but it was inexorable. The only drugs available to treat the disease had been around for more than 20 years; they improved some symptoms but did nothing to catch these slowly-falling souls. Accordingly, some national health insurance systems restricted or stopped paying for them.
In the last year, all this has fundamentally changed.
First came the ambiguity of a split decision between two clinical trials for the AmyloSENS therapy Aduhelm®/ aducanumab, followed by its controversial approval by the FDA. But then came an unambiguous success: in its Phase III clinical trial, lecanemab (Leqembi®) became the first therapy to unambiguously put the brakes on the trainwreck of neurodegenerative aging. The monoclonal antibody cleared beta-amyloid protofibrils from patients’ brains and slowed the downward plunge of the disease by some 27%, leading to FDA approval.
And now comes donanemab, a monoclonal antibody that for the first time specifically targets beta-amyloid that has been cut down to and modified into pyroglutamate at position 3 (pE3-Abeta). While we are still awaiting publication in a peer-reviewed journal, the press release from Eli Lilly tells us that over the course of a relatively short trial, donanemab passed the goalpost of slowing the progression of Alzheimer’s-type neurodegenerative aging (AD). That deceleration was measured on the integrated Alzheimer’s Disease Rating Scale (iADRS), meaning that people who received donanemab held onto their cognitive function and everyday mental engagement for longer. And in addition to iARDS, donanemab also put down the flaps on every other measure of cognitive and functional decline they tested.
And as we’ll discuss below, this top-line result actually understates how effective donanemab is — and a drill-down gives us a glimpse of a future where we can layer on additional rejuvenation biotechnologies to target other cellular and molecular damage in the aging brain.
This is a big deal. Beta-amyloid is one of the key forms of aging damage driving neurodegenerative aging of the Alzheimer’s type (AD), other neurodegenerative aging diseases, and so-called “normal” cognitive aging. Clearing out aggregated proteins like beta-amyloid has been part of the SENS strategy for ending human aging from the time that Dr. Aubrey de Grey first formulated the SENS approach. Antibody-based therapies to remove beta-amyloid from the brain were among the first rejuvenation biotechnologies to enter human clinical trials. And now they’re working, putting parachutes on people that aging processes had previously thrown ignominiously off the airplanes of life.
And yet some very sincere and serious advocates for our future are dismissing the results and suggesting that we need to look elsewhere. What gives?
The answer seems to be that they were not expecting a parachute, but a jetpack.
Now, anyone would prefer a working jetpack to even the best parachute. But let’s understand what humanity’s collective scientific investment has achieved here.
First, to repeat: aducanumab and lecanemab antibodies are the first FDA-approved rejuvenation biotechnologies, and donanemab is very likely soon to follow. All three directly remove molecular damage (beta-amyloid) from the aging brain. And for the first time in the terrible history of this ruinous consequence of the aging process, these therapies break the falls of people who have fallen off of the cruel cliff of AD.
Next consider the destruction that would already have rained down on the volunteers’ brains by the time they were eligible to enroll in the clinical trials for lecanemab and donanemab. All of the subjects had to be confirmed to have elevated beta-amyloid in their brains, and all had been diagnosed either with what is misleadingly called “mild” cognitive impairment, or with the earliest stage of AD.
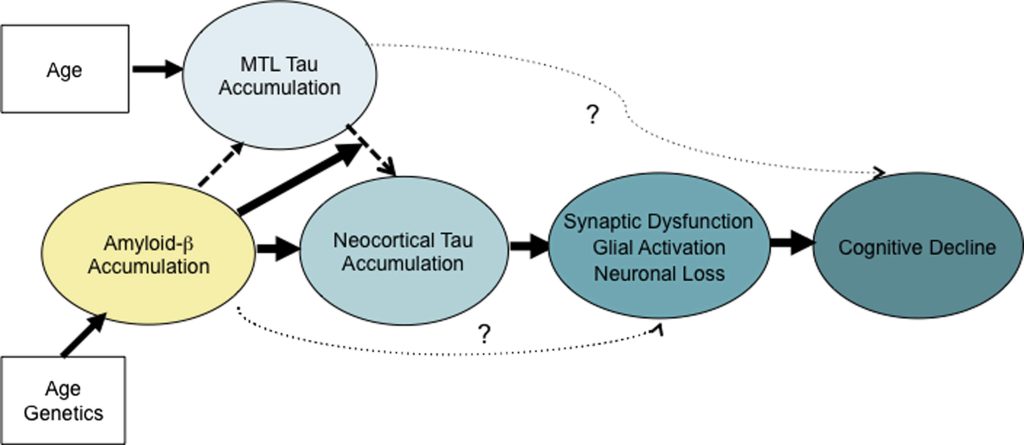
By the time a person reaches this level of cognitive impairment, such patients will also have had decades of abnormal forms of the protein tau accumulating inside the brain neurons in the medial temporal lobe and insinuating itself into the neocortex — the seat of memory and identity. This neuropathological invasion has been identified as critical in the transition to dementia as a downstream result of beta-amyloid accumulation.
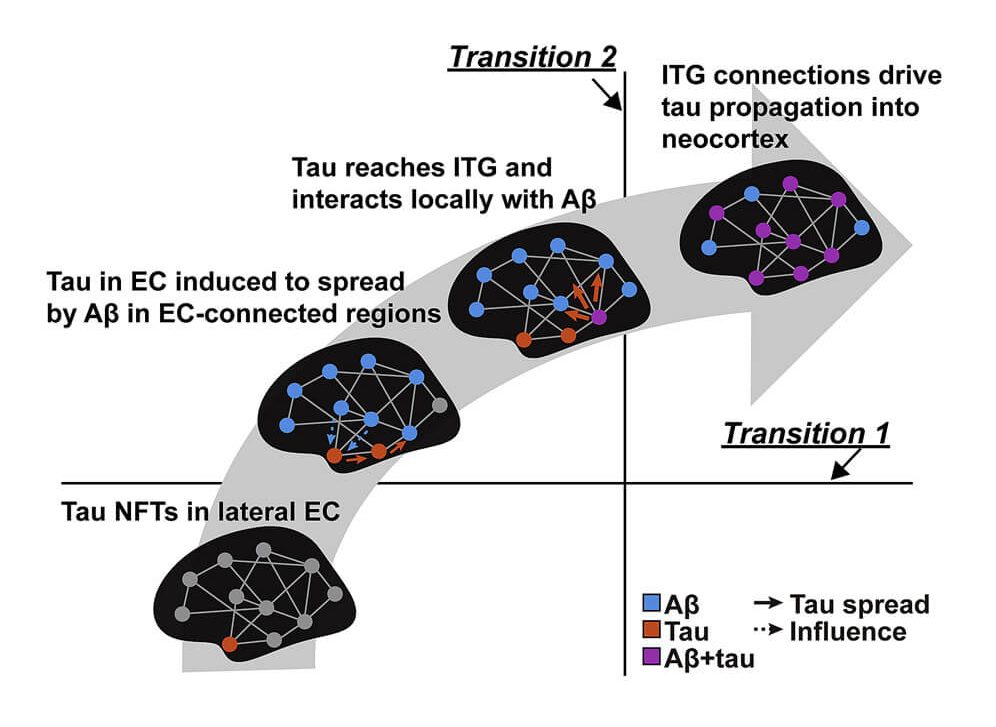
As we have only recently learned, people suffering with AD also have elevated numbers of senescent neurons in their brains — a burden even higher than that of age-matched control subjects. The same is true for the number of their neurons that have been hobbled by mitochondria bearing deletion mutations. And shockingly, they have already lost roughly a third of the neurons outright in the region of the brain that transfers short-term memory traces up from the hippocampus to the neocortex for long-term storage.
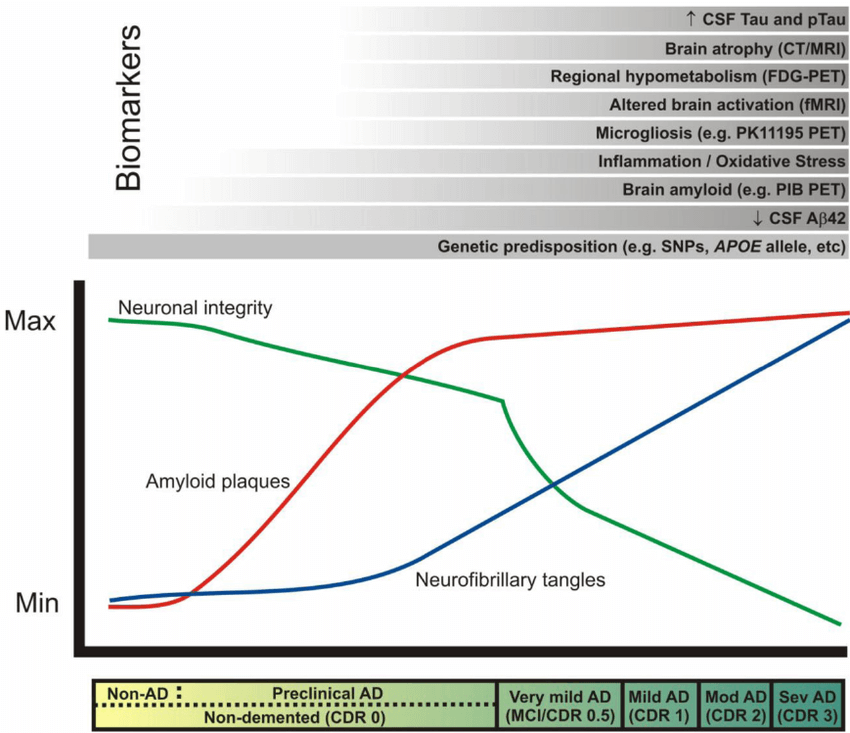
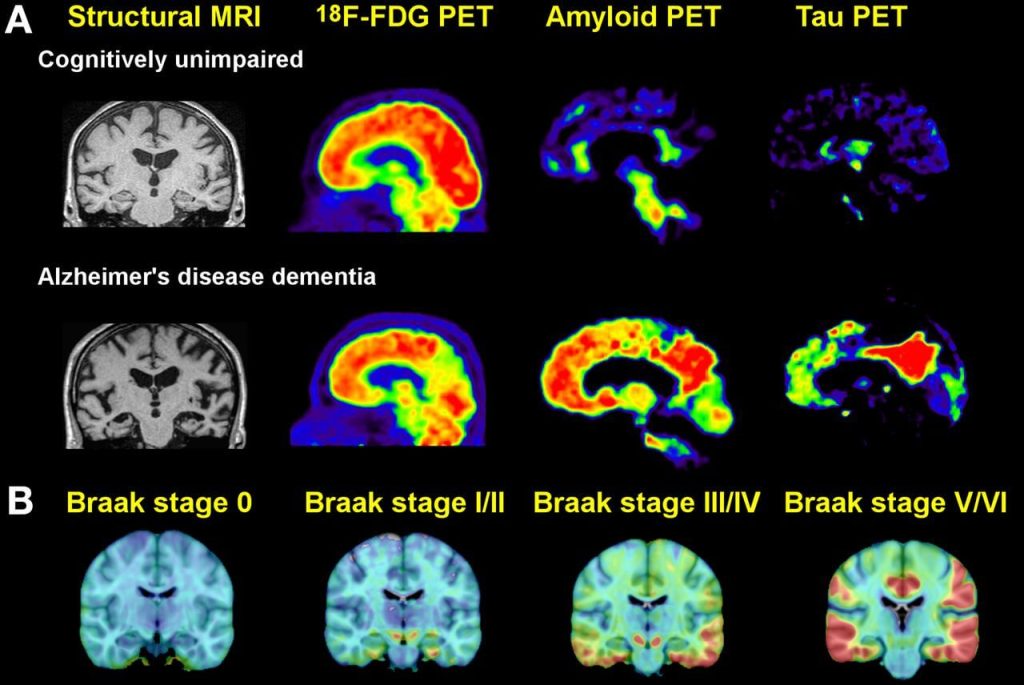
Again: all of this cellular and molecular carnage had been inflicted in the trial volunteers’ brains before they received even a single infusion of an AmyloSENS therapy. While clearing out their high burden of beta-amyloid will have allowed their surviving neurons to function better and reduced the rate at which new abnormal tau accumulated downstream, it could do nothing to remove all the non-beta-amyloid damage that had already in their brains. It’s frankly astonishing that removing just this one form of molecular damage from the brain can have such a profound effect when large numbers of their neurons are deranged in their function by abnormal tau, or senescence, or inflammatory attack by brain immune cells — or are long gone due to prior cell death.
How to Get There from Here
First Strategy: Head ‘Em Off at the Pass
The people expressing disappointment with the donanemab are absolutely right to insist that the ultimate goal should be to prevent AD from emerging — and to reverse it in its early stages if it does. As we’ve been highlighting for years, achieving this triumph will require the application of one of two “damage-repair” strategies. One approach is to begin removing beta-amyloid as soon as it starts to accumulate in the brain — meaning, in a person’s forties or fifties. This would prevent beta-amyloid from driving tau aggregates widely across the brain, and thus prevent the destruction of neurons downstream. It would also likely prevent some of the excess senescent cell burden in the AD brain and better-preserve brain mitochondrial function.
Of course, that is not how these medicines are being used today. But while the clinical trials for these AmyloSENS therapies didn’t start that early in the disease process, part of the reason they succeeded was exactly that they began treating patients earlier in the disease process than had been done in previous trials. Starting to clear the brain of beta-amyloid alone in people experiencing “mild” cognitive impairment or early-stage AD was still not an early enough start to head off the cascade of horrors in the AD brain entirely — but it was early enough that doing so could still have a substantial effect.
By contrast, in the previous Phase III trials of solanezumab, bapineuzumab, and other antibodies targeting beta-amyloid, the volunteers were all somewhere on the spectrum between so-called “mild” and so-called “moderate” AD dementia when they enrolled in the trial. This meant that in addition to having as much or more beta-amyloid in their brains from the get-go than was the case with the volunteers in the newer AmyloSENS therapies’ trials, the subjects in those earlier trials had already accrued substantially more non-beta-amyloid damage before they began receiving treatment, giving the antibodies much less of a chance to move the needle by preventing that downstream damage from accumulating. (I should clarify that this is just one of the many problems with the trials for sola and bapi — and regardless of trial design, there were also problems specific to solanezumab itself as a treatment).
Removing beta-amyloid when it first begins to meaningfully accumulate would prevent the invasion of aberrant tau into the neocortex and apare neurons from an aggregate-induced death. Brain immune cells could return to being neuronal guardians instead of being twisted into executioners; likely many cells could escape the insults that drive them senescent; and mitochondria that hadn’t suffered large deletions could keep functioning. This preemprive approach might delay neurodegenerative aging of the Alzheimer’s type by decades or longer, giving us time to develop the more comprehensive suite of rejuvenation biotechnologies that we will require to prevent or reverse it entirely.
Evidence from the Battlefield
This isn’t just an extrapolation from autopsy studies or lab animals with mutations knocked into them to make them accumulate the cellular and molecular damage of AD. Two clear lines of evidence from the clinical trials themselves support it.
The first is the relationship between the dose of these therapies, the magnitude of beta-amyloid clearance, and the cognitive benefit. Prior to the Phase III trials, all the now-triumphant anti-beta-amyloid antibodies underwent trials to determine the best dose to use.
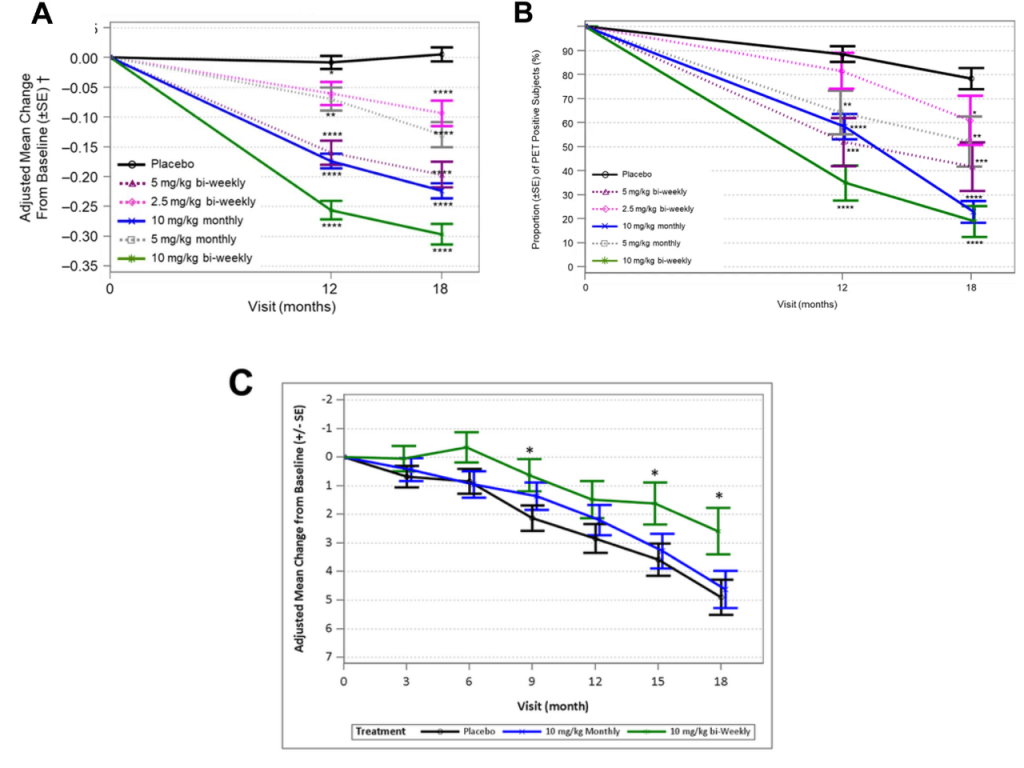
The higher the dose of antibody they administered and the more frequently they administered it, the more beta-amyloid lecanemab and donanemab cleared from the brain — and the more amyloid they cleared from the brain, and more they slowed the decline in cognitive function. That suggests that if we were to intervene earlier and prevent beta-amyloid from accumulating even to the level it had already accumulated in these patients when they first enrolled in the trial, the effect would be even stronger. And there’s even evidence to show that beta-amyloid clearance is more effective when the existing aberrant tau burden is lower. When they were negotiating the design of the Phase III trial of donanemab, Lilly scientists stipulated with regulators that they would pin their bets on the results in the subset of people who had “only” a moderate level of abnormal tau in their brains as measured on a PET scan, rather than high levels. You can probably guess why: based on everything we’ve just gone over, you would expect that a treatment that only removes beta-amyloid would be more effective in people whose brains had accumulated less rather than more downstream damage like aberrant tau.
It turned out that about two-thirds (1182) of the people who enrolled had such moderate levels, while the remaining third (552) had high levels. And when the results came in, the effect was exactly what that model would predict. On each and every measure of cognitive and functional decline, people who began the trial with a more moderate amount of aberrant tau got more benefit from beta-amyloid clearance with donanemab than did people who began with higher levels.
| Intermediate Tau-Only Group | Intermediate & High Tau Group |
| iARDS | 40% | 23% |
| CDR-SB | 36% | 29% |
| ADCS-iADL | 43% | 28% |
| ADAS-Cog13 | 35% | 19% |
People who began the trial with less pre-existing aberrant tau in their brains got more benefit from beta-amyloid clearance with donanemab than did people with higher tau burdens. iADRS = Alzheimer’s Disease Rating Scale; CDR-SB=Clinical Dementia Rating—Sum of Boxes; ADCS-iADL = Alzheimer’s Disease Cooperative Study instrumental Activities of Daily Living (iADL) Inventory; ADAS-Cog13 = the 13-item Alzheimer’s Disease Assessment Scale – Cognitive subscale. Data from Eli Lilly Press release, May 3, 2023
To realize the full potential of removing beta-amyloid alone, you would want to begin treatment before the cascading destruction downstream of beta-amyloid had set in earnest — before people even began questioning whether they might be starting to slip. Happily, after the success of lecanemab in Phase III, the National Institutes of Health joined forces with Esai (the AmyloSENS therapy’s maker) and decided to take this next logical step. Together, they have launched the AHEAD Study. This clinical trial will test lecanemab in volunteers with no evidence of cognitive impairment, but who have either intermediate (in one trial) or higher (in another) levels of beta-amyloid in their brains. The goal will be to see if starting at this early stage can keep people free from ever developing MCI and dementia in the first place.
Second Strategy: All the Damage, All the Time
If the first strategy is to hit beta-amyloid as early as possible so as to keep the downstream damage from forming in the first place, the second strategy is for when it’s too late for that. When a person not only has a substantial amount of beta-amyloid in her brain, but also tau in her neocortex, substantial neuronal loss in key regions of the brain, and so on, simply removing beta-amyloid can only be expected to do so much. And remember: that was exactly the condition of all the patients in these newer trials. All of this downstream damage is already well-established by the time a person even has “mild” cognitive impairment, let alone any level of clinical AD dementia.
What, then, would it take to take a person with the level of damage to the brain that the people who enrolled in these Phase III trials had and go beyond slowing down further progression and into actually stopping the disease in its tracks — or reversing it? Achieving that much more ambitious goal would entail going after all that damage directly. Beta-amyloid clearance will still be a central tool in the toolkit — and doing so can potentially be made safer and more effective by replacing the current generation of binding (IgG) antibodies with catabodies. Catabodies are catalytic antibodies that cleave their targets directly where they encounter them instead of having to bind to them and drag them to the circulation for eventual disposal via the liver or passive degradation.
Catabodies offer the potential of both more bang for the buck (because each molecule can degrade multiple molecules of their target instead of having to bind to and tow their target molecules out of the brain one by one) and of greater safety (since they break up their target on site, instead of having to drag it out through the brain’s vasculature, where it may cause damage and inflammation. Indeed, the damage inflicted by wrenching beta-amyloid out of the brain is the most worrisome risk associated with the current beta-amyloid targeting antibodies, taking the form of vascular swelling and microbleeds. Catabodies targeting beta-amyloid may offer a safer and more effective way to clear the brain of this central player in AD.
Since we would be intervening after beta-amyloid had already had the chance to drive aberrant tau into the neocortex, we would also need to clear aberrant tau from inside neurons. Several therapies targeting aberrant tau are in clinical trials right now; unfortunately, they all target these aggregates outside the neurons. Such tau is easier to access, but it is not the pool of tau that does the most damage. And while removing extracellular tau is likely to interrupt the spread of aberrant tau into even more neurons, it won’t do much or anything to rescue the neurons that it has already seeded. SENS Research Foundation scientists are right now working on a project to remove aberrant tau within neurons, using a novel delivery system to get the antibody inside the cell and catabodies to destroy the junk right there on the spot, thus dispensing with the need to drag it out of the cell and then out of the brain.
And since critical areas of the brain have already suffered substantial neuronal attrition in critical areas by the time a person experiences MCI, we’ll also need to replace those neurons with new ones, and reinforce the rest of our neuronal circuitry to ensure against further losses. Stimulating the birth of new neurons from the stem cells already present naturally in the brain (neurogenesis) and providing trophic factors like brain-derived neurotrophic factor (BDNF) won’t do the job because only a very restricted area of the adult human brain is even capable of neurogenesis, and most of those neurons only support the formation of new memories, not our ability to hold onto existing memory, identity, and function. Again SRF is on the case, funding Dr. Jean Hébert’s innovative neuronal replacement strategy to disperse replacement cells widely across the brain.
As we mentioned, the AD brain is also riddled with senescent cells, so destroying those dysfunctional cells will also be essential to extending our cognitive lifespans. Readers will know that drugs that do this in mice have profound rejuvenating effects in mice — including in animal models of Alzheimer’s disease. And as we speak, senolytics are being tested in multiple human clinical trials for specific diseases of aging — including neurodegenerative aging of the Alzheimer’s type. The main downside of these drugs is collateral damage. They work by inhibiting pathways on which senescent cells disproportionately rely for their survival — but some perfectly normal cells also lean heavily on these pathways, leading to potentially life-threatening destruction of blood clotting cells by navitoclax and the destruction of macrophages (a kind of immune cell) by navitoclax, dasatinib plus quercetin (D+Q), and fisetin. Dr. Amit Sharma’s team at SRF is pursuing what is likely to be a more selective strategy, harnessing the evolved capacity of the immune system to destroy senescent cells.
And finally, in addition to the ways that beta-amyloid hobbles our brain’s energy-generating mitochondria, even more neurons are overtaken by mitochondria bearing deletion mutations in the AD brain than in the brains of other people of the same age, in whom there are already far too many. So rendering mitochondrial deletions harmless is another way to keep the spark of life firing in our neurons — and SRF scientists are working on this too.
Go Early or Go Broad to Go Home
So yes: beta-amyloid clearance can only go so far in patients whose brains are as damaged as the volunteers in the trials of lecanemab and donanemab. But granted that damage — and the prior history of attempts to bend the terrible curve of neurodegenerative aging of the Alzheimer’s type — the effects were a smashing success. Even when the earlier trial for what now appears to be a significantly less effective therapy (aducanumab) was halted early, the patients knew they were benefitting and wanted back in.
So we celebrate this victory — and we press on to repair all the damage of aging, journeying ever closer to our future home free of the degeneration of body and mind.