To date, all successful interventions into the biological aging process in experimental animals have entailed modulation of basic metabolic pathways, generally through genetic or dietary manipulation.(0) Of these, the earliest, most well-studied, and arguably the most robust, is Calorie restriction (CR): the reduction in dietary energy intake, without compromise of essential nutrients.(1,2) With few exceptions, CR retards the biological rate of aging in nearly every species and strain of organisms in which it has been tested, ranging from rotifers, through small multicellular invertebrates, and most extensively to laboratory rodents; and although inconclusive, recent evidence also supports its effectiveness in dogs(3) and nonhuman primates.(4) Moreover, while necessarily preliminary, a growing body of human research has reported that rigorous CR, when practiced by previously normal-weight adults, results in physiological, functional, and perhaps even structural changes consistent with its translation to the human case.(5-8)
While by no means universal (eg. (9,10)), there is therefore widespread optimism in the biogerontology community that CR would be similarly effective in humans. The most vocal such investigator was UCLA’s Dr. Roy Walford, who was the first of small number of human prolongevists (of which the present author is one exemplar) to be sufficiently impressed by this evidence as to take up long-term, rigorous CR themselves, in hopes of enjoying the longevity and protection against age-related disease and disability observed in other species. Some members of the nonprofit CR Society, cofounded by Dr. Walford, are CR practitioners, and some of these are the subjects of one the most informative of the human CR studies.(eg, (5-8)).
This widespread optimism as to the translatability of CR, however, is tempered by equally widespread pessimism that any significant number of humans will take it up in practice. Instead, the focus of biogerontologists’ thinking surrounding the potential clinical implications of CR research has long been to identify the core mechanism(s) responsible for the extension of youthful health and lifespan by CR, and to then target those mechanisms with “CR mimetics:” small molecules that would induce the “anti-aging” effects of CR, while neither requiring nor resulting in any reduction of energy intake. The CR mimetic concept was first formally formulated by Lane, Ingram, Roth of the National Institute on Aging (NIA) in 1998(11) and has been a subject of growing interest ever since (eg, (2,12)).
But despite the initial attractiveness of the notion; its strong theoretical basis; the high level of scientific interest that it has garnered; the launching of biotech startups originating in CR mimetic research; and the popularization and commercial exploitation of the concept by the dietary supplement industry — despite all of these drivers, the ensuing decade and a half or more of CR mimetic research have thus far been fruitless. Initially-promising compounds have failed to extend lifespan, while surprising findings have preempted the further investigation of what might otherwise have been novel targets for CR mimetics. Here we review some of the more prominent cases.
Antioxidants - Free Radicals
The free radical theory of aging — both in its early, simplistic form, and in its later and more refined iterations — and the support afforded to it by the CR data, will be familiar to readers of this forum, and need not detain us. The same is likely true of intervention with dietary antioxidants, which have repeatedly been the subject of testing in animal models and in human clinical trials, and which have repeatedly failed to (respectively) extend lifespan in normal, healthy mammals, or to improve clinical outcomes in humans at risk for disease. These elementary facts bear brief mention for completeness; having made such allusion, we will now move on to less well-known disappointments.
2-deoxyglucose (2DG) - Nutrient Sensing
This was the first agent to be formally investigated as a putative CR mimetic; indeed, the initial report of these studies marked the first formulation of the concept and the coining of the term “CR mimetic” itself.(11) 2DG, an unmetabolizable analog of glucose, exhibits similar pharmacokinetics as the parent molecule, including in its cellular uptake and initial entry into the glycolytic pathway — properties that had previously been exploited to probe glycolysis itself, and as a radioactive tracer for PET scanning. Following its initial metabolism by hexokinase, further metabolism of 2DG is arrested, and it therefore acts as a competitive inhibitor of cellular glucose metabolism. Lane et al’s interest in this compound were based on prior reports indicating that 2DG induced physiological and bioenergetic effects parallel to CR, including lowering body temperature, altering reproductive function, and even inhibiting tumor growth. Their own initial studies showed that 2DG feeding also lowered serum glucose and insulin levels in rats, while causing only relatively minor reductions in body weight;(11) later studies by their group and others found that 2DG lowers heart rate, elevates circulating glucocorticoids and expression of heat shock proteins (effects thought to underlie a protective “hormetic” mechanism of CR), increases resistance to cold shock, affordins substantial protection against ischaemic and toxic insults to the brain, and increases mean and apparently maximal lifespan in Caenorhabditis elegans (reviewed in (14)).
But mammalian lifespan studies of 2DG proved initially frustrating, and ultimately futile. A first study at doses that had induced a CR-like metabolic pattern in rats led to premature deaths associated with congestive heart failure and cardiac vacuolization, while lower dosages sufficient to avoid cardiac toxicity failed to alter metabolism and had no effect on lifespan.((11-14); Figure 1). Later studies using alternative material sources (to rule out a possible role for contaminants) and different strains of rat confirmed the compound’s cardiotoxicity, with histopathology additionally finding pheochromocytoma in 2-DG-fed rats.
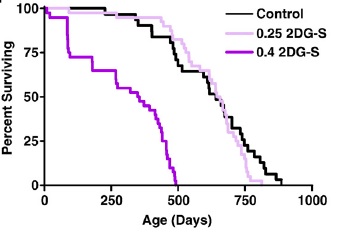
Figure 1. Survival of F344 rats on 2 doses of 2-deoxyglucose. Reproduced from (14).
Metformin - Insulin Signaling, AMPK, Gene Expression
CR robustly increases insulin sensitivity, lowers blood glucose, and reduces the risk of diabetes in rodents and nonhuman primates, and of impaired glucose tolerance in humans. These effects have long been postulated to be central to the retardation of aging by CR.(15) Metformin, an hepatic gluconeogenesis inhibitor and mild insulin sensitizer used in the treatment of diabetes, emerged as a widely-cited possible CR mimetic as a result of these effects, along with its activation of AMPK, the wide overlap of its effects on hepatic gene expression profiles with those of CR, and a possible reduction in mitochondrial free radical generation due to inhibition of Complex I of the electron transport chain.(16) These mechanistic findings were bolstered by the protective effects of related biguanide drugs in genetic models of carcinogenesis; by the reduction of mortality in a short-lived mice when administered metformin itself; and by the uniquely protective effects of metformin (against total and cancer mortality observed in diabetic patients.(16) But as reviewed elsewhere, the long-delayed results of a careful life extension study using metformin in F344 rats have recently published, finding no effect — although there was sufficient ambiguity in the results the issue cannot yet be said to be fully resolved.(17) Separate studies on metformin are underway in the lab of Dr. Steven Spindler, UC Riverside.
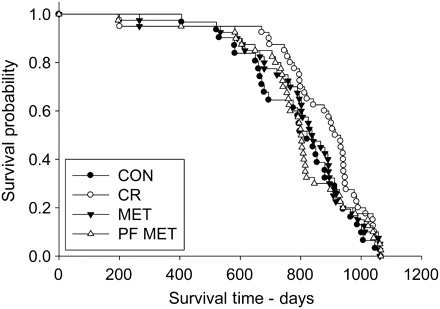
Figure 2. Kaplan–Meier survival plots for CON, CR, MET, and PF–MET (CON: n = 31; CR: n = 40; MET; n = 40; and PF–MET: n = 40). Reproduced from (17).
Pimagedine - Glycation and Advanced Glycation Endproducts
Along with its reduction of circulating glucose and triglycerides, CR has repeatedly been found to reduce the age-related accumulation of advanced glycation endproducts (AGE) in the tissues of animals. Due to the implication of AGE in the complications of diabetes and the age-related tissue dysfunction, this structural effect of the altered metabolism of fuels by CR has been specifically highlighted as a potentially central mechanism of CR.(16) The rate of accumulation of the markers of glycation and glycoxidation in tissue collagen was the only promising candidate biomarker of aging to emerge in relation to the NIA’s Biomarkers of Aging program, and was specifically found to be a limiting factor for the individual survival of both CR and ad libitum mice. (18) In two studies unfortunately marred by animal cohorts with historically low longevity, the length of life was greater when fed a low-AGE chow,(19) while high-AGE chow blunted of the extension of life by CR.(20)
Pimagedine (aminoguanidine), a drug once under investigation as a therapeutic for the complications of diabetes, is a competitive inhibitor of AGE formation, sequestering reactive dicarbonyl intermediates and retarding the rate of accumulation of AGE in the tissues of rodents, and was the subject of much interest as a possible longevity therapeutic and partial CR mimetic. However, it failed to meet its primary outcome in clinical trials in diabetic subjects (creatinine doubling time) and was associated with a range of mild to moderate adverse reaction,(21) and rodent studies carried out by Dr. Spindler found no benefit in normal, nondiabetic mice.(16)
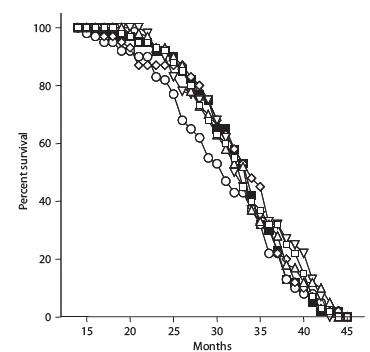
Figure 3. Survival of mice administered control diet alone (solid black square), or with aminoguanidine (downward pointing triangle; 65 mg/kg body weight/d), aminoguanidine and alpha-lipoic acid (hollow diamond; 65 and 73 mg/kg. respectively), aminoguanidine, alpha-lipoic acid, pregnenolone, and coenzyme-Q 10 (hollow circle; 65, 73, 0.2, and 12 mg/kg), melatonin (hollow square, 41 µg/kg), or melatonin and pregnenolone (upward pointing triangle, 41 and 200 µg/kg). Reproduced from (16).
Resveratrol - Sirtuins
By far the most well-publicized possible CR mimetic ever was the phytoalexin polyphenol resveratrol, present in trace amounts in grapes and (famously) wine. Interest in resveratrol was initially sparked by research on Sir2, an NAD+-dependent histone deacetylase in the baker’s yeast Saccharomyces cerevisiae. Reports in S. cerevisiae, C. elegans, and Drosophila melanogaster indicated that lifespan could be extended in these organisms by 30–50% by increased copy number or expression of the gene; because its activity was reponsive to the cellular NAD+:NADH ratio, and because of reports that life extension by CR-like dietary manipulation in S. cerevisiae and Drosophila required Sir2, it was hyypothesized that Sir2 activation might be a key mechanism of CR. Investigation of resveratrol as a CR mimetic began with a report that it was one of a small number of “sirtuin activating compounds (STAC)” identified in a screen using recombinant human SIRT1 (the human homolog) in vitro, and the hypothes was underscored by reports that administration of resveratrol extended life in the same range of lower organisms as had already been reported to respond to Sir2. Nearly all of these claims were later disputed,(22-27) but significant public and scientific interest had already been generated, and experiments in mice were initiated.
Interest increased dramatically by reports that high-dose resveratrol supplements partially normalized lifespan(28) and various aspects of health and functionality(28,29) in mice made obese and diabetic by a high-hydrogenated-coconut-oil diet. In the popular press, to a limited degree in the scientific literature, and especially in dietary supplement companies’ promotional materials, these results were often misconstrued as demonstrating actual extension of normal, youthful functionality and lifespan, leading to remarkably widespread interest and enthusiasm.
But finally, in 2008, the results of a lifespan study of 3 doses of resveratrol in normal, healthy mice were published.(30) While some specific aspects of age-related deterioration were retarded in resveratrol-fed mice, survival and pathology were unaffected. Surprisingly, this negative result has had virtually no effect on media coverage, and mention of the result is (unsurprisingly) studiously avoided in promotional material.
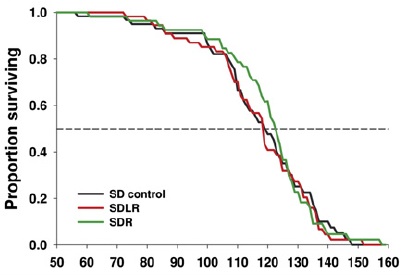
Figure 4. Effects of resveratrol on mice fed standard diet (SD) or SD plus a low (100 mg/kg food = 7.9 ± 0.2 mg/kg body weight, SDLR) or high (400 mg/kg food = 30.9 ± 0.6 mg/kg wt) dose of resveratrol. “Later, additional groups of mice were given a higher dose of resveratrol along with the standard … diets (2400 mg/kg of food, SDHR) … beginning at 12 months of age (SDHR) on lifespan, and again found that longevity was not significantly affected.” Reproduced from (30).
Additional studies are underway to test resveratrol at several doses and with 2 ages of initiation through the NIA’s Interventions Testing Program, “a multi-institutional study investigating treatments with the potential to extend lifespan and delay disease and dysfunction in mice.”
Rapamycin - mTOR
Finally, however, 2009 saw the publication of a successful CR mimetic. Rapamycin is an inhibitor of the mammalian Target of Rapamycin (mTOR), a highly conserved protein kinase serine/threonine kinase that integrate signals from nutrients (cellular ATP and amino acid levels) and trophic signaling (insulin/insulin-like growth factor 1 (IGF-1) and other mitogens) to regulate autophagy, cell growth, and cell cycle progression. Rapamycin (sirolimus /Rapamune®) and a range of analogs are therefore already FDA approved cancer treatments, immunosuppressants, and cardiology drugs, and additional analogs are in development.
Inhibition of TOR has emerged in recent years as a strong candidate mechanism of CR, due to its involvement in cellular response to energetic and trophic signaling, its inhibition during CR in a wide range of animal models, and the extension of life in several such models garnered by disrupting its signaling pathway.(31) It was therefore put into testing in the NIA’s ITP. And after a series of disappointments with other modulators of putative metabolic mediators of biological aging, these studies led the programto an historic first. Rapamycin is now the first pharmaceutical intervention to robustly extend the normal lifespan of genetically intact, well-husbanded mammals (genetically heterotgeneous mice) of both genders.(32) Even more impressively, it was shown to be effective when administered relatively late in life (600 d, vs. historical and cohort control mean and maximum lifespans of ~900 d and 1100-1200 d, respectively, for mice).
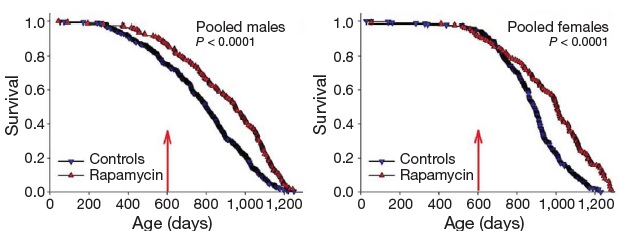
Figure 5. Effect of late-life rapamycin administration on survival in mice. Reproduced from (32)
The result being rightly heralded for the landmark advance in biogerontology it undoubtedly is (it received an honorary Mprize Lifespan Achievement Award), it none the less illustrates the limitations of the CR mimetic approach. While the relative gain in mean lifespan (remaining life expectancy at first exposure to rapamycin (600 d)) was substantial (28% for males, 38% for females), the absolute gains were relatively modest: 9% and 13%, respectively (and similarly for tenth-decile survivorship).(32) CR itself, when implemented in longevous male at a similar age, has been reported to increase maximum longevity by ~16%(33).
These gains are enormous, when compared to the multiple null results or even harmful effects reported in previous studies, they are in absolute terms relatively small — and certainly smaller than the ~40% increases in mean and maximal lifespan gained when CR is initiated shortly after weaning. And it is to these latter, far higher figures that advocates of the CR mimetic approach often refer when making their case (eg. 34):
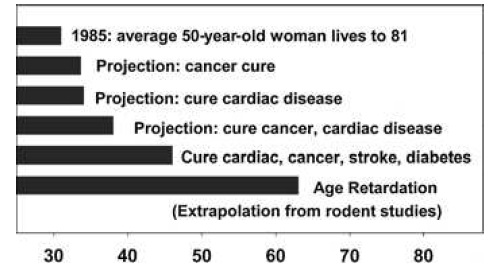
Figure 6. “Remaining life expectancy of a 50-year-old Caucasian woman in the United States in 1985, at then-current mortality risk schedule (top bar), or as projected under the assumption that adult mortality risks for specific diseases (cancer, cardiac disease, etc., as indicated) were reduced to zero from 1985 onward. The bottom bar shows projected life expectancy if human adult mortality risks could be reduced to the same extent that caloric restriction reduces them in mice.” Legend and figure reproduced from (34).
… despite the obvious fact that there is no prospect, for ethical reasons, of such an intervention protocol ever being carried out in humans. It is rather in persons of middle age and above that even the most liberal of regulatory and oversight bodies would allow a CR mimetic to be administered, supposing them to already be in reasonable good health for their age. It is thus to the more modest figures for late-life intervention (whether by CR or proper) that reference must be made to make a case that accurately reflects the underlying data.
The support lent the CR mimetic approach by the rapamycin result must also be tempered by a comparison of the results of this study (32) to the effects of CR itself. While one must be cautious in comparing results acquired using different protocols and in different laboratories, it is fully as one would expect that the lifespan gains reported for rapamycin are significantly less than those reported for CR in the same gender and initiated at the same age (9% vs ~16%). The more modest reported effect of rapamycin has several plausible and somewhat trivial explanations, which are not mutually exclusive, including differences in rodent strain, husbandry, and other protocols, and especially the optimization of the intervention dose. But one that should be highlighted, and would be expected to apply on a priori grounds to even the most rigorously-matched set of experimental conditions, is that it is unreasonable to expect that a pharmacological mimetic of CR will be as effective as CR itself. Leaving aside the potential limitation of the benefits of any drug therapy by off-target effects, it seems unlikely that all of the pleiotropic effects of CR but one (such as inhibition of mTOR signaling) are dispensable to its ability to extend the healthy, youthful lifespan.
Is it reasonable to expect, for example, that the full contribution to the retardation of biological aging by CR afforded by reductions in insulin, IGF-1, cellular amino acids, and ATP are mediated by downstream inhibition of mTOR? Is it not more plausible to expect that these effects of CR contribute to the modulation of aging through additional, unrelated mechanisms? And what of the many physiological effects of CR that do not affect the mTOR pathway at all, such as the reductions circulating glucose, lipids, and lipoproteins, body temperature, sex steroids, and visceral adiposity? Are we to think that the modulation of these multiple disease- and aging-associated parameters by CR is fully dispensible to the extension of youth, supposing only that mTOR activity is optimally suppressed? Rather, it seems more reasonable to expect that the only intervention that will fully deliver the sought-for gains in healthy lifespan derived from CR, will be CR itself.* All pharmacological mimics will, in this analysis, be expected to be shadows of CR itself, of greater or lesser opacity.
Beyond CR Mimetics
Recognizing the inherent limitations of the CR mimetic concept as a strategy to develop therapeutic interventions against degeneration of biological aging, regenerative engineering is advanced as an alternative. Rather than attempting to modulate basic metabolic pathways in hopes of reducing the deleterious side-effects that they produce, regenerative engineering proposes to develop a new class of therapeutics that directly remove, repair, replace, or render harmless the cellular and molecular damage that accumulates in tissues over time, impairing functionality and resulting in the progressive rise in frailty, disease, disability, and death that people now suffer with advancing age. With the burden of such damage removed from aging tissues, their structure and in principle functionality would be restored, leading to the renewal of youthful health and vigor. And because this strategy does not necessitate the modulation of metabolic pathways to be effective, regenerative engineering therapeutics should be less prone to generating the deleterious side-effects that must inevitably accompany interference with the biochemical basis of life itself. SENS Foundation is dedicated to accelerating the development of a comprehensive suite of regenerative engineering interventions, to prevent and even reverse the degenerative aging process in as many persons as possible, on the most aggressive possible schedule.
* On the other hand, it also bears mention that it is equally unreasonable to think that all of the physiological effects of CR are necessary contributors to its benefits. If so, then a CR mimetic that bypassed some such effects might deliver (some of) the benefits of CR while dispensing with (some of) its undeniable deleterious or troublesome effects.(35)
References
0. Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008 Aug 28;454(7208):1065-71. Review. PubMed PMID: 18756247; PubMed Central PMCID: PMC2774752.
1. Weindruch R., Walford R. L. The Retardation of Aging and Disease by Dietary Restriction. 1988; Charles C. Thomas Springfield, IL.
2. Smith DL Jr, Nagy TR, Allison DB. Calorie restriction: what recent results suggest for the future of ageing Research. Eur J Clin Invest 2010; 40 (5): 440-50.
3. Kealy RD, Lawler DF, Ballam JM, Mantz SL, Biery DN, Greeley EH, Lust G, Segre M, Smith GK, Stowe HD. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc. 2002 May 1;220(9):1315-20. PubMed PMID: 11991408.
4. Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009 Jul 10;325(5937):201-4. PubMed PMID: 19590001; PubMed Central PMCID: PMC2812811.
5. Cangemi R, Friedmann AJ, Holloszy JO, Fontana L. Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell. 2010 Apr;9(2):236-42. Epub 2010 Jan 20. PubMed PMID: 20096034.
6. Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr). 2010 Mar;32(1):97-108. Epub 2009 Nov 11. PubMed PMID: 19904628; PubMed Central PMCID: PMC2829643.
7. Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008 Oct;7(5):681-7. PubMed PMID: 18843793; PubMed Central PMCID: PMC2673798.
8. Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007 Aug;42(8):709-12. Epub 2007 Mar 31. Review. PubMed PMID: 17482403; PubMed Central PMCID: PMC2020845.
9. de Grey AD. The unfortunate influence of the weather on the rate of ageing: why human caloric restriction or its emulation may only extend life expectancy by 2-3 years. Gerontology. 2005 Mar-Apr;51(2):73-82. Review. PubMed PMID: 15711074.
10. Shanley DP, Kirkwood TB. Caloric restriction does not enhance longevity in all species and is unlikely to do so in humans. Biogerontology. 2006 Jun;7(3):165-8. PubMed PMID: 16858629.
11. Lane MA, Ingram DK, Roth GS. 2-Deoxy-D-glucose feeding in rats mimics physiologic effects of calorie restriction. J Anti-Aging Med. 1998 Winter;1(4):327-37.
12. Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary Interventions to Extend Life Span and Health Span Based on Calorie Restriction. J Gerontol A Biol Sci Med Sci. 2010 Apr 6. [Epub ahead of print] PubMed PMID: 20371545.
13. Lane MA, Mattison J, Ingram DK, Roth GS. Caloric restriction and aging in primates: Relevance to humans and possible CR mimetics. Microsc Res Tech. 2002 Nov 15;59(4):335-8. Review. PubMed PMID: 12424798.
14. Minor RK, Smith DL Jr, Sossong AM, Kaushik S, Poosala S, Spangler EL, Roth GS, Lane M, Allison DB, de Cabo R, Ingram DK, Mattison JA. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol. 2010 Mar 15;243(3):332-9. Epub 2009 Dec 22. PubMed PMID: 20026095; PubMed Central PMCID: PMC2830378.
15. Masoro EJ, McCarter RJ, Katz MS, McMahan CA. Dietary restriction alters characteristics of glucose fuel use. J Gerontol. 1992 Nov;47(6):B202-8. Erratum in: J Gerontol 1993 Mar;48(2):B73. PubMed PMID: 1430849.
16. Spindler SR, Mote PL. Screening candidate longevity therapeutics using gene-expression arrays. Gerontology. 2007;53(5):306-21. Epub 2007 Jun 15. Review. PubMed PMID: 17570924.
17. Smith DL Jr, Elam CF Jr, Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB. Metformin Supplementation and Life Span in Fischer-344 Rats. J Gerontol A Biol Sci Med Sci. 2010 Mar 19. [Epub ahead of print] PubMed PMID: 20304770.
18. Sell DR, Kleinman NR, Monnier VM. Longitudinal determination of skin collagen glycation and glycoxidation rates predicts early death in C57BL/6NNIA mice. FASEB J. 2000 Jan;14(1):145-56. PubMed PMID: 10627289
19. Cai W, He JC, Zhu L, Chen X, Wallenstein S, Striker GE, Vlassara H. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expression. Am J Pathol. 2007 Jun;170(6):1893-902. PubMed PMID: 17525257; PubMed Central PMCID: PMC1899464.
20. Cai W, He JC, Zhu L, Chen X, Zheng F, Striker GE, Vlassara H. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. 2008 Aug;173(2):327-36. Epub 2008 Jul 3. PubMed PMID: 18599606; PubMed Central PMCID: PMC2475771.
21. Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P, Ratner RE, Spinowitz BS, Whittier FC, Wuerth JP; ACTION I Investigator Group. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004 Jan-Feb;24(1):32-40. Epub 2003 Dec 17. PubMed PMID: 14685005
22. Garber K. A mid-life crisis for aging theory. Nat Biotechnol. 2008 Apr;26(4):371-4. PubMed PMID: 18392009.
23. Ledford H. Ageing: Much ado about ageing. Nature. 2010 Mar 25;464(7288):480-1.
24. Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell S, Napper A, Curtis R, Distefano PS, Fields S, Bedalov A, Kennedy BK. Substrate specific activation fo sirtuins by resveratrol. J Biol Chem. 2005 Jan 31; [Epub ahead of print] PMID: 15684413
25. Zou S, Carey JR, Liedo P, Ingram DK, Müller HG, Wang JL, Yao F, Yu B, Zhou A. The prolongevity effect of resveratrol depends on dietary composition and calorie intake in a tephritid fruit fly. Exp Gerontol. 2009 Jun-Jul;44(6-7):472-6. Epub 2009 Mar 3. PubMed PMID: 19264118.
26. Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007 Oct;128(10):546-52. Epub 2007 Aug 14. PubMed PMID: 17875315.
27. Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010 Mar 12;285(11):8340-51. Epub 2010 Jan 8. PubMed PMID: 20061378; PubMed Central PMCID: PMC2832984.
28. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006 Nov 16;444(7117):337-42. Epub 2006 Nov 1. PMID: 17086191 [PubMed – indexed for MEDLINE]
29. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006 Dec 15;127(6):1109-22. Epub 2006 Nov 16. PMID: 17112576 [PubMed – indexed for MEDLINE]
30. Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008 Aug;8(2):157-68. PMID: 18599363 [PubMed – as supplied by publisher]
31. Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009 Oct;1790(10):1067-74. Epub 2009 Jun 16. Review. PubMed PMID: 19539012.
32. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009 Jul 16;460(7253):392-5. Epub 2009 Jul 8. PubMed PMID: 19587680; PubMed Central PMCID: PMC2786175.
33. Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci U S A. 2004 Apr 13;101(15):5524-9. Epub 2004 Mar 25. PubMed PMID: 15044709; PubMed Central PMCID: PMC397416.
34. Miller RA. Extending life: scientific prospects and political obstacles. Milbank Q. 2002;80(1):155-74. PMID: 11933792 [PubMed – indexed for MEDLINE]
35. Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006 Jan;127(1):1-7. Epub 2005 Oct 13. Review. PubMed PMID: 16226298.



