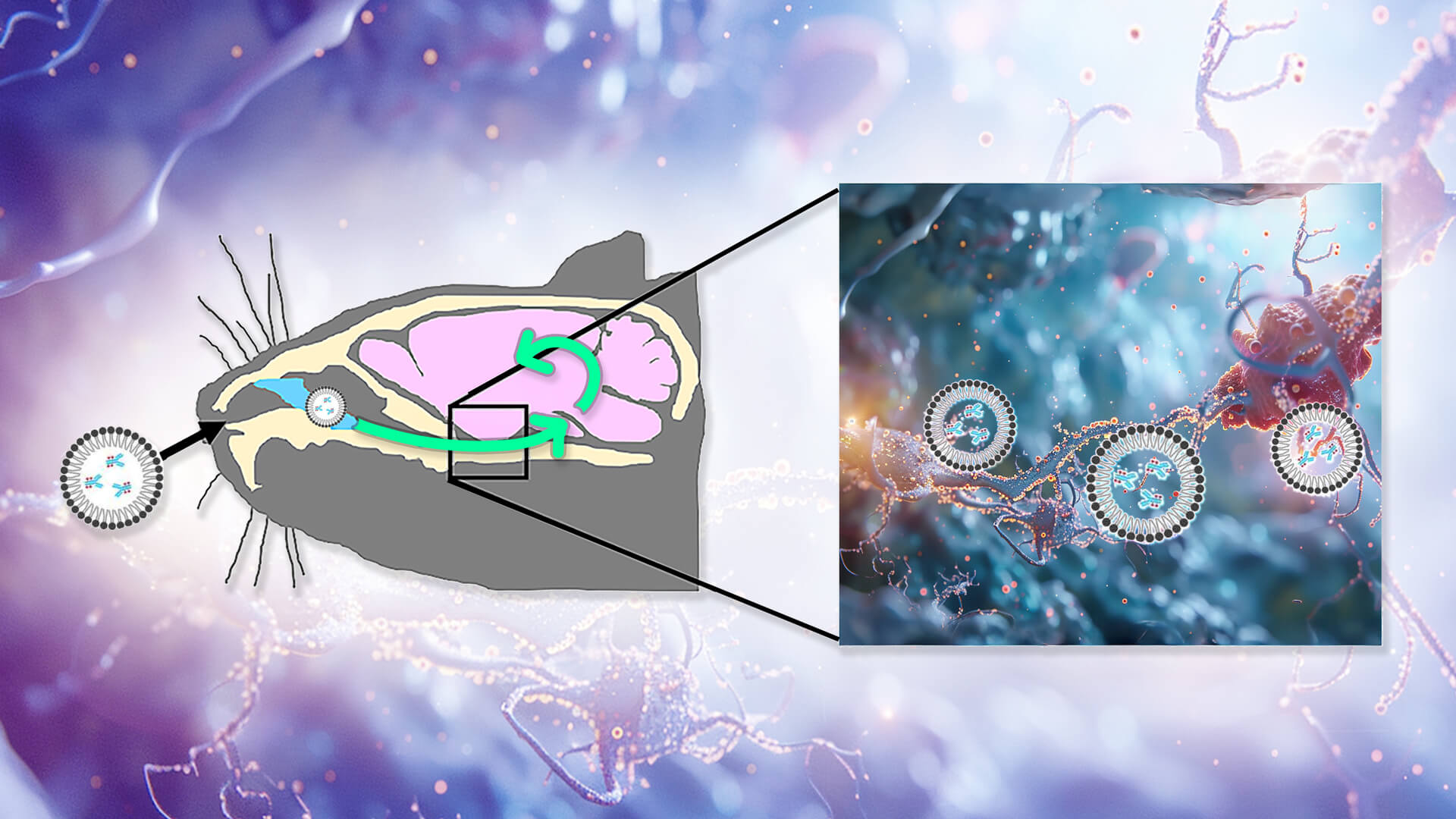
TRIMming Tau Damage in Its Foxholes
Aberrant tau inside neurons is a key driver of Alzheimer’s disease, but nearly all the therapies in development to target it can only capture the small amount that floats outside of them. A new animal study reports impressive results in clearing aberrant tau inside neurons and rejuvenating cognitive function, opening up an important new front in damage-repair strategies for maintaining the aging brain.