A message from Edward James Olmos
Why is COVID-19 so deadly to the elderly?
As most people know, the greatest predictor of death from this pandemic is age. The so-called comorbidities predisposing patients to death from COVID-19 — chronic lung diseases, damaged kidneys and hearts, high blood pressure, diabetes — are themselves aspects of aging, erupting in their distinctive ways in particular tissues. Flattening this “demographic curve” of degenerative aging would reduce COVID-19 to a disease similar in impact to an average recent flu season (and make future flu seasons less deadly), while also putting an end to the staggering toll of age-related death and debility that ticks on in the background even now, day in and day out, pandemic or none.
Ending that toll is our mission. At SENS Research Foundation (SRF), we develop rejuvenation biotechnologies: new therapies that will repair the accumulated cellular and molecular damage in our tissues and restore youthful function.
The pandemic was a call to action. It demonstrates the critical need for better long-term strategies for addressing threats to human life. As members of the global scientific community, all of us at SRF acknowledge the need to adapt and apply our expertise and experience to the current crisis. (SARS-CoV-2 is the coronavirus that causes the disease called SARS-2 or COVID-19.)
Below, we outline some of the ways in which specific forms of aging damage are relevant to diseases like COVID-19 – and how some of our research programs may help render this and other viruses far less dangerous in the future.

Aging’s effect on the COVID-19 mortality rate, and the anticipated effects of future rejuvenation biotechnologies
Data taken from Eur J Epidemiol 2020 Dec 09. doi: s10654-020-00698-1.
Age-flattened curve represents the decrease in COVID-19 fatality that could result from breakthroughs in rejuvenation biotechnology.
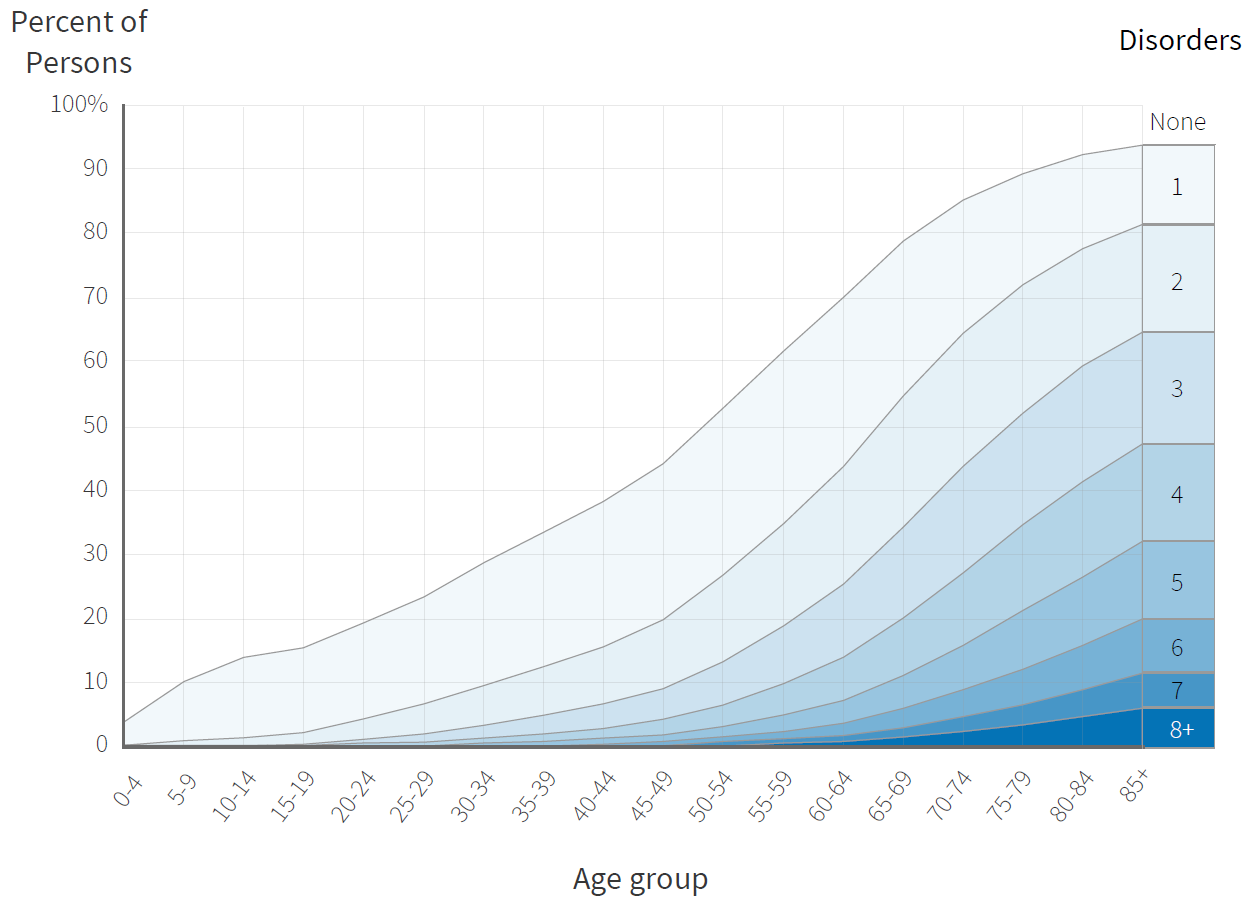
Comorbidities by age group.
Data taken from Lancet 380(9836):37-43.
Ending that toll is our mission. At SENS Research Foundation (SRF), we develop rejuvenation biotechnologies: new therapies that will repair the accumulated cellular and molecular damage in our tissues and restore youthful function.
The SARS-CoV-2 pandemic is both an immediate, pressing danger, and a call to action. It demonstrates the critical need for better long-term strategies for addressing threats to human life. As members of the global scientific community, all of us at SRF acknowledge the need to adapt and apply our expertise and experience to the current crisis. (SARS-CoV-2 is the coronavirus that causes the disease called SARS-2 or COVID-19.)
Below, we outline some of the ways in which specific forms of aging damage are relevant to diseases like COVID-19 – and how some of our research programs may help render this and other viruses far less dangerous in the future.
Rejuvenate the Immune System
Strong and early T-cell responses appear to be critical to the immune system’s ability to successfully fight off SARS-CoV2. CD8+ (“killer”) T-cells, and other parts of the immune system fight viruses by eliminating cells that are infected with the virus, preventing them from becoming factories to churn out more infectious particles. In a study of 207 COVID-19 patients tracked from their first reported symptoms, researchers found that those who mounted an early, robust killer T-cell response avoided inflammation setting in across their bodies and suffered only mild or asymptomatic disease, whereas a delay in mounting such a response was linked to system-wide inflammation at the very beginning of symptoms and was followed by hospitalization. Similarly, two studies showed impaired cytotoxic T-cell function in older COVID-19 patients specifically might be causally linked to severity of the infection (PMID: 32948688, 32463803). A meta-analysis revealed lower counts of CD4+ and CD8+ T cells may contribute to severity of infection and are correlated with poor prognosis (PMID: 32598880). In addition to reduced numbers, a retrospective analysis in patients requiring hospitalization compared to healthy controls shows a strong correlation with T cells with markers of exhaustion (like PD-1 and Tim-3) in patients requiring hospitalization (PMID: 32425950).
The loss of immune function with age also impacts the vaccine campaign. Thanks to the aggressive work of scientists around the world and robust funding from the United States, Germany, and other governments, we are now benefitting from vaccines against SARS-CoV-2 that are safer and more effective than most scientists dared to dream in the early days of the pandemic, and these have rightly been prioritized for older adults in recognition of their extreme risk of illness and death from the disease. But even when using potent COVID-19 vaccines, we see that degenerative aging greatly weakens the immune response.
Moderna’s clinical trial reported its COVID-19 vaccine to be 96% effective in preventing disease in adults under age 65, but “only” 86% effective in people 65 and older — still powerful protection, but aging took its toll. And it’s reasonable to assume that they would have seen further declines in efficacy with age if they had broken down the over-65s into subgroups of older and older people.
The picture with Pfizer’s vaccine is more complicated. Both the main clinical trial of Pfizer’s COVID-19 vaccine and real-world data from the CDC confirms two weeks after receiving their final vaccine dose, younger and older subjects have equal (and powerful) protection against severe illness and death. But the initial immune response lags in older people, leaving them with a window of greater risk. This has important consequences in Canada and numerous European countries, where governments have attempted to make the best use of limited vaccine supplies by delaying the administration of the second vaccine doses in order to get first shots into more arms faster. A study designed to test the effects of this policy found that elderly nursing home residents produce far fewer antibodies — and especially the critical neutralizing antibodies — in response to that first dose of the Pfizer COVID-19 vaccine than younger healthcare workers working in the same centers. Similarly, real-world data from Israeli healthcare workers found that the immune response to Pfizer’s COVID-19 vaccine weakened with age.
These studies highlight that older people mount a much weaker and less complete immune response to both infections and to vaccines, even as they suffer increasingly from overactive parts of the immune response, including autoimmunity and chronic inflammation.
Two key parts of this immunosenescence (aging of the immune system) are waning production of naïve T-cells by the aging thymus and damage to the lymph nodes, such that the lymph nodes are no longer able to keep emerging T-cells alive, functional, and ready for future threats. SENS Research Foundation has sponsored several projects aimed at developing damage-repair technologies to restore aging T-cell numbers and function, including pilot studies of a T-cell scrubber that might clear out a specific class of dysfunctional T-cells and early-stage work toward a tissue-engineered thymus, along with a pilot animal study to simulate the effects of both interventions.
In addition to CD8+ T-cells, COVID-19 patients suffer from an exhaustion of natural killer (NK) cells (PMID: 33154753). Whereas T-cells and B-cells are specialists, focused on eliminating specifically-identified threats (such as cells infected with specific viruses), NK cells are sentinels patrolling the perimeter of a military camp, on the lookout for anything that looks like it doesn’t belong. NK cells attack abnormal cell types such as cancer cells, cells infected by viruses like SARS-CoV-2, and senescent cells — that is, cells that have undergone changes that prevent them from replicating, and that spew out a witches’ brew of inflammatory signaling molecules, growth factors, and enzymes that break down proteins. This brew is called the senescence-associated secretory phenotype, or SASP.
Long before the pandemic hit, we knew that NK cells lose much of their effectiveness with age, meaning that aging people already come into the fight against infections like SARS-CoV-2 with these critical early responders weakened. At our Research Center, Dr. Amit Sharma and his team have been developing strategies to rejuvenate and reinforce NK cells in aging people. They recently collected preliminary data showing that the proportion of NK cells exhibiting markers of strong cell-killing ability declines sharply with age. To confirm this preliminary finding, they will look for an age-related reduction in NK cells’ ability to kill senescent cells, using NK cells freshly isolated from young adult, middle-aged, and older people. They will run parallel tests on NK cells from the spleens of young (6 months) and old (24 months) mice. Moving from basic research to anti-aging intervention, the team is developing strategies to enhance senescent-cell-killing ability in old NK cells. They will test rejuvenation strategies including adoptive transfer of young NK cells into aging mice, and agents that sidestep the protective shielding that senescent cells throw up to defend themselves against NK cells.
If transferring young NK cells works as a proof of concept, it would support moving forward by adapting immune transfer biotechnologies already in use for cancer therapies to instead selectively target senescent cells. In CAR-T cell therapy for some cancers, a patient’s T-cells are drawn out with the blood, expanded in number, and engineered to express artificial CAR receptors. These receptors specifically target proteins found on the surface of the cancer cells, and the T-cells can also still attack cancer cells that are no longer displaying markers that T-cells normally need to identify and attack. These CAR-T cells are then re-infused into the patient to attack the cancer aggressively.
Recently, CAR-T cells were engineered to target senescent cells, zeroing in on a receptor that scientists tentatively identified as commonly displayed by them. At the same time, CAR engineering of cancer patient cells has been used with NK cells. This CAR-NK cell technology is very new, but has already revolutionized immunotherapy for some cancers at MD Anderson and elsewhere, and thirteen clinical trials are underway in other cancers, including some against which CAR-T therapy has not (or has not yet) proven effective. And NK cells — not T-cells — are the natural immunological enemies of senescent cells. So by combining NK cells’ intrinsic senescent cell-stalking abilities with CAR receptors laser-focused on markers displayed on the senescent cell surface, the SRF team expects to generate a remarkable chimeric predator specialized in eliminating these cells.
Purge Senescent Cells
For Younger Lungs...
Some of the rejuvenation strategies being tested by Dr. Sharma’s laboratory will likely enhance aging NK cells’ ability to eliminate any kind of abnormal cell, including those infected by SARS-CoV-2. But the SENS lab is focused on rejuvenating the capacity of NK cells to eliminate senescent cells because of their broad role in driving aging pathology, and it’s not a coincidence that many of their ill effects directly impact a person’s vulnerability to COVID-19.
First is senescent cells’ role in driving fibrosis in our tissues. Multiple aspects of lung function decline with age, while fibrosis increases. Accordingly, diseases of the lung — including chronic obstructive pulmonary disease (COPD), lung cancer, and most especially idiopathic pulmonary fibrosis (IPF) — are profoundly age-related. Preliminary evidence suggests that the lung is one of the organs most burdened with senescent cells in aging in humans — a burden further exacerbated by IPF.
We’ve known for a while that the age-related loss of lung function is a massive driver of risk of death from pneumonia. Aging people not only have fewer functional alveoli available, but progressively lose the ability to inhale and exhale deeply to compensate for alveoli taken offline by the infection. Continuing research suggests that eliminating senescent cells in the lung may preserve and restore youthful lung function, leaving the lungs better prepared to endure the attack of the SARS-CoV-2 virus and other causes of pneumonia.
Senolytic drugs, which selectively kill senescent cells, have been shown to reverse lung fibrosis and other tissue fibrosis in aging mice. Studies in aging mice with inbuilt “suicide genes” demonstrate that ablating senescent cells in aging mice restores youthful lung compliance, suggesting an opportunity to do the same with other senescent-cell elimination strategies, such as restoring the ability of NK cells to eliminate them from tissues. Further supporting this, lung fibrosis is partially reversed by two different senolytic drugs in mouse models of IPF, and a third senolytic partially reversed lung fibrosis in mice whose lungs have suffered radiation damage.
... and a Rejuvenated Signaling Environment...
In addition to lung damage, another way that senescent cells may exacerbate COVID-19 involves the SASP cocktail of inflammatory factors and proteins that degrade the network of proteins that support the organs in which they’re embedded. Some researchers have argued that inflammatory factors in the SASP may also suppress the immune response to the virus underlying COVID-19 (SARS-CoV-2). This hypothesis is based on a number of previous studies showing that chronic inflammation caused by numerous different conditions interferes with the immune response to multiple other viruses, including blunting the immune system’s response to vaccines against influenza, yellow fever, and hepatitis B. This is another aspect of the research currently being undertaken at SRF. To keep the immune system healthy to fight new infections, we are developing novel ways to remove or sequester pro-inflammatory SASP that builds up in serum with age and blunts the immune response to viral infections like SARS-CoV-2.
Inflammation driven by macrophages in the lesions of patients with atherosclerosis suppress the activation of T-cells, and this is associated with the failure of T-cells from these patients to mount an effective T-cell response against the virus that causes chickenpox in children and shingles (herpes zoster) in older adults. In one study, damping down the release of inflammatory factors in the skin before administering a shingles vaccine virus boosted the T-cell response to the vaccine.
Inflammation is complicated, however: acute inflammatory responses to injury or infection are essential to wound repair and successful immune response, respectively, whereas the chronic inflammation of aging impairs both, drowning out the local ramp-up when immune cells are actually needed and instead dispersing those cells all over the body to sites riddled with aging damage, futilely trying to repair microscopic injuries they cannot resolve. This is why drugs and antibody therapies that simply force down the inflammatory response lead to vulnerability to infection.
The solution here is not to attack the inflammation, but to remove and repair the underlying damage of aging, thereby eliminating the source of chronic inflammatory stimulus while freeing up the rejuvenated tissues’ ability to mount an effective inflammatory response to acute threats.
A surprising example of this has emerged in the context of aging and COVID-19. As a result of the pandemic, people worldwide have become familiar with the cytokine storm — a severe inflammatory response that leads to immune derangement and the deadly acute respiratory distress syndrome (ARDS) that directly kills so many COVID patients. Cytokine storms are also involved in many other viral fatalities, and the fact that young people can mount aggressive cytokine storms is thought by many scientists to be the reason why so many middle-aged people were killed by the 1918 influenza epidemic, even though flu normally stalks the elderly and young children while leaving middle-aged people alive.
But there’s a wrinkle on cytokine storms and aging in COVID-19. Chinese researchers have found that a delayed immune response to the virus, as much as the strength of it, predicts death from COVID-19, accompanied by higher levels of inflammatory factors at death and depleted levels of multiple immune cell types. A study in aging monkeys suggests reasons why. The researchers found young monkeys infected with SARS-CoV-2 quickly mounted a savage immune response, complete with extensive attack of macrophages and T-cells and high levels of inflammatory factors within the first week of infection, but were quickly able to recover after that. By contrast, the immune response was delayed in old monkeys — and this seemed to have cost them. Having gotten started late, the old animals’ immune systems seem to have attempted to make up for lost time, mounting a more severe cytokine storm that recruited even higher levels of infiltrating macrophages and drove a more persistent T-cell attack. Yet those aged T-cells were also less effective at actually fighting the virus, making the inflammation and immune cell attack on the tissues purely self-destructive — a story we have seen play too often in our hospitals.
One important component of the SASP is an inflammatory factor called IL-6, which rises with age and predicts the risk of frailty and death even without SARS-CoV-2 infection. A report suggests that a hospitalized COVID-19 patient’s IL-6 level is a strong risk factor for eventually requiring a ventilator, suggesting that senescent cells make aging people more vulnerable to the disease, and that senescent cell ablation could shore up this vulnerability. These findings are so compelling that some clinical centers treating critically ill COVID-19 patients are making experimental use of monoclonal antibody therapies such as tocilizumab and sarilumab, which block IL-6’s access to its receptors. However, several trials of tocilizumab in COVID-19 patients had been completed as of April 2021, with mixed results and the suggestion that it may be beneficial only to some groups of patients or in combination with glucocorticoids. But if we restore NK cells’ ability to eliminate senescent cells, people infected with SARS-CoV-2 would start off with lower IL-6 levels more characteristic of a young person, and thus better prepared for the fight.
In addition to IL-6, it’s recently been discovered that there is a network of factors emitted in the SASP that trigger the formation of blood clots and impede the countervailing factors that dissolve them. It’s long been known that an imbalance in these factors becomes increasingly common as people age, especially if they have risk factors for cardiovascular disease. The discovery that the SASP could tip the balance toward excessive coagulability, combined with the fact that aging people’s tissues become increasingly riddled with senescent cells over time, suggests that senescent cells and their SASP may be a key driver of this process.
Senescent cells’ possible culpability in the pro-clotting bias in aging people’s blood was already an important avenue for research before the rise of COVID-19, since the excessive tendency to form and maintain clots puts them at greater risk of heart attack, stroke, and venous thromboembolism (VTE) — abnormal clots forming in the veins. But it becomes a matter of acute focus in the face of multiple reports that high levels of markers of excessive clotting are common in COVID-19 patients at hospitalization, and foreshadow admission to the ICU and death from or with COVID-19 (in Holland and in Wuhan). Indeed, despite receiving prophylactic anti-clotting medication, nearly a third of Dutch patients with COVID-19 suffered from dangerous blood clots, including very commonly VTE that work their way up to cut off the lung tissue’s own blood supply, starving the lung itself of oxygen even as it is under attack by the virus and the patient’s own immune system.
Medical researchers have suggested a number of possible causes of excessive clotting specific to COVID-19, but as usual, the role of aging itself has been almost entirely ignored, despite the powerful influence of age in one’s risk of dying of the disease. Older people’s burden of senescent cells, the recent research suggests, may be predisposing them to a clotting crisis if infected by SARS-CoV-2.
Fortunately, the same research that originally identified the pro-clotting cocktail in the SASP also suggests that rejuvenation biotechnology could eliminate the associated risk of dangerous blood clots. Mice, like people, suffer a rise in senescent cell burden when given the chemotherapy drug doxorubicin, and the cells release SASP factors that favor the formation and stability of blood clots. In response, the mice produce higher levels of clot-initiating platelets, and those platelets are placed on a hair trigger. Activating a senescent-cell-destroying suicide gene prevented all of these things from happening, suggesting that purging aging cells from aging people could also leave them better prepared to survive an infection with SARS-CoV-2. Conversely, researchers at the Mayo Clinic have discovered that proteins from the SARS-CoV-2 virus exacerbate the SASP in human senescent cells, creating a vicious cycle of inflammation consistent with the ravages of the virus in older people.
... and Now in Human Trials
Work is already underway to translate these exciting results into human rejuvenation therapies. Mayo Clinic researchers last year conducted a very early-stage clinical trial of drugs that trigger self-destruction of senescent cells in human patients with IPF. Although there were few clearly apparent benefits to senolytic therapy in this study, it was too short-term and involved too few patients (just 14) to expect anything obvious: happily, the researchers are working to expand this pilot study into a larger clinical trial, and other such trials are underway in patients with kidney disease and osteoarthritis, diseases also driven by senescent cells. We will soon begin seeing what these therapies can do to maintain our health and resilience against the forces of degenerative aging and COVID-19.
In fact, there’s now proof-of-concept evidence that eliminating senescent cells can protect the body against mouse beta-coronavirus — the same subgroup of coronaviruses to which SARS-CoV-2 belongs. Mayo Clinic scientists recently found that administering a senolytic agent allows mice to survive infection with mouse beta-coronavirus. The evidence is so compelling — and the intensity of the pandemic so threatening — that they are conducting a clinical trial of a senolytic for older people hospitalized with COVID-19, aiming to keep them from drowning in the abnormal age-related cytokine storm.
Trigger Self-Destruction of Mutation-Prone Cells
More than half of the human genome is invasive genetic data left behind by viruses, including millions of retrotransposons. Retrotransposons are “dead” DNA, but their long- and short- interspersed virus-like repetitive elements (LINEs and SINEs) encode machinery that —under certain circumstances — allows them to reactivate, replicate, and spread through the genome. These reactivation events can cause mutations in our functional genes and even disrupt the normal expression of non-mutated genes, leading to cancer, cellular self-destruction (apoptosis), and cellular senescence.
To develop a proof of concept for a new class of “retrolytic” drugs that would ablate these cells before they can further damage the body, SENS Research Foundation is sponsoring work by Dr. Andrei Gudkov and his team at the Roswell Park Comprehensive Cancer Center for a suicide-gene system similar to the groundbreaking INK-ATTAC system that paved the way for the senolytic revolution. As a side-benefit, the gene whose expression will activate the retrolytic suicide gene is also activated in cells with active viral infection (such as SARS-CoV-2), which may eliminate such cells before they are hijacked by the virus to replicate itself.
Transplant Mitochondria to Rescue Critical Lung Cells
Several gene-expression and protein distribution studies demonstrate that the ACE2 receptor — the critical loophole through which the SARS-CoV-2 virus slips into our cells — is more enriched in a type of lung cell known as AT2 cells, and COVID-19 patient autopsy reports indicate that these cells are subject to a terrible assault during the disease. AT2 cells are critical support cells for type I alveoli — the tiny air sacs that expand and contract to effect gas exchange and respiration. AT2 cells produce the pulmonary surfactant that allows type I alveoli to expand again after contraction by reducing alveolar surface tension. This surfactant also facilitates the exchange of gases between the oxygen-poor, CO2-enriched venous blood and the relatively oxygen-rich air in the lungs; we believe the virus’s assault on these cells is a major contributor to respiratory failure.
It’s these same AT2 cells that fail in an animal model of septic pneumonia, and these mice are rescued by transplanting bone marrow stem cells that donate their mitochondria to the failing AT2. Dr. Amutha Boominathan, Nana Anti, and Lauren Kirk of our mitochondrial mutation rescue team have been developing our mitochondrial transplantation protocol. Their initial target is different, but we hope it will treat many conditions of acute energy depletion, as is already being done in small open clinical trials for babies with heart damage from ischemia-reperfusion injury.
A New Generation of SENS Scientists in a New Model System
Last summer, SRF partnered with Dr. Evan Snyder at the Sanford Consortium for Regenerative Medicine to learn more about COVID-19. Under the expert mentorship of the Snyder lab, six of our SRF Summer Scholars successfully established innovative lung and brain organoid models as well as a lung epithelium model to uncover how SARS-CoV-2 infects these organs, investigate drugs that might treat or ameliorate the disease, and look for clues about what makes aging people more vulnerable. These models contain blood vessels, since abnormal clotting and damage to blood vessels are critical vulnerabilities in COVID-19. The lung epithelial model also offers the potential to identify age-related or virus-induced damage to specialized lung cells’ ability to produce the critical surfactant needed to keep lung air sacs functional. Our Scholars demonstrated that these systems could be infected with the virus and that they could use them to test drugs that might thwart that infection and to profile aspects of COVID-19 that might make the elderly so vulnerable (including increased inflammation, compromised vasculature, loss of critical lung proteins, and aging brain cells). The progress achieved by our Summer Scholars over such a short time prompted SRF to extend one Summer Scholar for an extra quarter of research to do a deep dive into perfecting the model system to show that blood vessels that compose the lung may be particularly vulnerable to aging-related changes, especially those mediated by inflammation, but may be amenable to increasing surfactant production. Taken together, these studies established model systems that may accelerate progress toward novel therapies against COVID-19 and against lung and brain aging.
Conclusion
Like the pandemic, aging touches all of us. It creeps silently through our tissues, progressively crippling our minds and bodies, and eventually killing us if we don’t die first of accident, violence, or other abrupt age-independent causes. In COVID-19, the damage caused by aging is the largest factor in determining who lives and who dies, even if the trigger was pulled by a virus spread by globalization. The need for rejuvenation biotechnologies as part of medicine has never been clearer, and so we strengthen our resolve. Restoring our cells and tissues to youthful vigor will allow us to step out of our ancient lockdown and into a bright future.
Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy.
Onder G, Rezza G, Brusaferro S.
JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4683. [Epub ahead of print] PubMed PMID: 32203977.
The dramatically higher case-fatality rate for COVID-19 in Italy as compared with anywhere else in the world (including in the epicenter of the pandemic in Wuhan, China) has been the subject of much speculation and concern. These investigators find the phenomenon to be almost entirely explained via the age structures of the populations, with a much larger share of persons aged >65 in Italy. Looking within each age group, the case-fatality rates in Italy and China are highly comparable for all persons >65, but were higher in Italy in persons age >70 and even more so in those >80 years; these numbers are skewed, however, by the particularly low number of people in these age groups in China as a share of persons >65, and especially the lack of any patients aged >90 in the Chinese reports, who were at very greatly increased risk of death in Italy.
Convalescent plasma transfusion for the treatment of COVID-19: Systematic review.
Rajendran K, Narayanasamy K, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A.
J Med Virol. 2020 May 1. doi: 10.1002/jmv.25961. [Epub ahead of print] Review. PubMed PMID: 32356910.
Convalescent plasma is a very old therapy, first used for diphtheria in the late nineteenth century. Doctors often turn to it for new infections in the absence of established medical therapy, and this has been the case in COVID-19. Available studies appear generally favorable, but are all very small and have no control group, and the evidence for convalescent plasma in most diseases is weak; it was used successfully to treat Ebola, but in a randomized trial of 140 children and adults, it was found no more effective against influenza than control plasma from uninfected subjects. Importantly, it did seem to have salutary effects in the original SARS virus, including some controlled trials; however, none of the trials were of high quality. Three randomized controlled trials are underway in the United States to test the intervention: one to protect medical workers who are not yet infected from the disease, and two in hospitalized patients at different stages in the disease progression.
A key limitation of the therapy is the low ratio of recovered patients compared to those needing therapy; if it works, it could be the basis for a monoclonal antibody therapy, which could be scaled up to treat far more patients and could likely be made more effective. It would also provide some preliminary confidence for the possibility of a vaccine, whose prospects are uncertain at this time. Fortunately there has been no evidence of antibody-dependent enhancement (ADE) in the use of convalescent plasma for either SARS or COVID-19, although ADE was observed in animal models of SARS and in response to an experimental SARS vaccine in nonhuman primates.



