Hello! My name is Sheri Zada. This past June, I completed a Bachelor of Science degree in Neuroscience and a minor in Bioinformatics at the University of California, Santa Cruz. Prior to my participation in the SENS Research Foundation Summer Scholars Program, I had been working in the Hinck Lab at UCSC for over a year to determine the Slit/Robo signaling pathway’s role in breast cancer. There is strong evidence that the Slit/Robo signaling pathway is involved during metastasis in the mammary gland. Under the supervision of Dr. Lindsay Hinck and Dr. Gwyndolen Harburg, I investigated the Slit/Robo signaling pathway in cancerous stem cells. The intrinsic property of stem cells is their ability to self-renew. SLIT is a tumor suppressor protein, and its binding to the ROBO receptor has downstream effects in the cell cycle. A cellular mechanism for such an effect is aging or senescence, defined by the inability to engage in cell division. In this mechanism, a stem cell loses its intrinsic ability to self-renew and becomes senescent.
I utilized a human cell line derived from a human mammary carcinoma to observe the effects of SLIT treatment on cancerous cells. The cell line exhibits stem-cell like properties. Therefore, in vitro studies of these cells induced into senescence could assist me in developing an understanding of the behavior of human mammary stem cells in vivo. By increasing Slit/Robo signaling, I altered the cell cycle of these cancer cells in vitro. This was evidenced through counting colonies of cells in cell culture and observing the numbers over time. To further analyze cell cycle behavior, I checked if the cells had undergone senescence by using an assay called senescence associated β-galactosidase. This assay identifies senescent cells by their ability to hydrolyze a specific substrate. The cells undergoing senescence express the enzyme that reacts with this substrate and produce a precipitate that stains the cells blue. Thus, the senescent cells are visible to the human eye. By initiating Slit/Robo signaling in MDA-MB-231 cells, I observed an increase in the rate of aging as measured by the induction of senescence and, subsequently, a decrease in self-renewal as measured by the cell-culture studies. My contributions to the mammary stem cell studies conducted in Dr. Hinck’s lab will be published in Stem Cell Reports this year.
As a SRF Summer Scholar, I will be spending the next 12 weeks in Dr. Afsie Sabokbar’s research lab, which is located in the University of Oxford’s Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences (NDORMS).
Identification of SCARA5, a Novel Biomarker in Bone Pathophysiology
With guidance from Dr. Sabokbar and Dr. Hussein Al-Mossawi, I am examining molecular pathways implicated in diseases affecting the rudimentary structure of a human being, osteoarthritis, and rheumatoid arthritis. The research team endeavors to seek high efficacy treatment for those suffering from bone diseases by characterizing novel molecular targets involved in bone remodeling. Humans have built-in homeostatic mechanisms in their bone marrow for maintaining the balance of bone formation and resorption by way of two cell types, osteoblasts and osteoclasts. The two regulate each other but possess different origins. Osteoblasts differentiate from mesenchymal stem cells, which are located in the bone marrow. Also found in the bone marrow, are hematopoietic stem cells that give rise to immune cell types, one being the monocyte/macrophage lineage. Differentiated from monocytes, a macrophage can be induced by cytokine signals to form osteoclasts. Therefore, the monocyte/macrophages may be possible participants in bone synthesis or resorption.
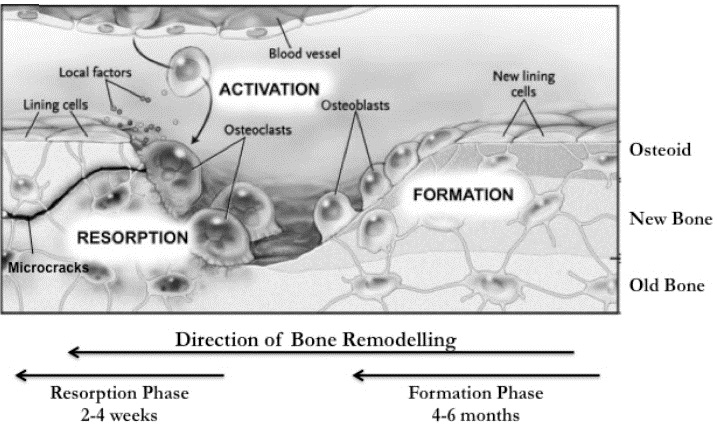
Figure 1. A comparison of osteoclast and osteoblast function.
This figure identifies the phases and identified players in bone synthesis (osteoblasts) and resorption (osteoclasts). Figure courtesy of Kular et al., 2012.
Class A macrophage scavenger receptor (SR-A) is predominantly expressed in macrophages/monocytes, and, thus, its cell lineage drives its potential importance in bone turnover (Lin et al., 2007). The signaling of SR-A is critical for osteoclastogenesis. In SR-A knockout mice, researchers observed a 50% reduction of the osteoclast population, thus displacing the normal bone turnover rate and allowing high levels of osteoblastogenesis (Lin et al., 2007). Understandably, these mechanisms produced a high bone density phenotype. A relative to SR-A is a receptor being studied in the Sabokbar lab called scavenger class A receptor member 5 (SCARA-5). In SCARA-5-/- mice, bone density was high in comparison to wild-type mice, thus implying a role in the bone remodeling processes (Jiang et al., 2006). The lab has previously identified SCARA-5 products in mouse trabecular bone in the joint and continues to investigate SCARA-5 in human tissue. However, the precise mechanism(s) for SCARA-5 function still remains unclear. By collecting human osteoarthritic and rheumatoid arthritic tissue and synovial fluid samples, SCARA-5’s role in human joint diseases can be assessed.
I am undertaking various cohesive studies in the Sabokbar lab for a comprehensive analysis of SCARA-5’s role in bone regulation. Currently, no specific biomarkers for osteoarthritis and rheumatoid arthritis exist. The level of SCARA-5 in a patient’s joints could possibly be used by clinicians to detect an onset of osteoarthritis or rheumatoid arthritis. In liaison with clinicians, I will use rheumatoid arthritis and osteoarthritis patient-donated samples to test for SCARA-5 levels. These samples contain bone tissue, which I plan to stain for SCARA-5 in immunohistochemistry experiments. Samples of synovial fluid taken from the joint will be used to compare the amount of SCARA-5 found in patients suffering from different bone diseases. Further, samples of whole blood from patients will be used to determine SCARA-5 expression in various immune cells that mediate bone remodeling. I aim to contribute to the ongoing studies of bone turnover processes in the Sabokbar lab and, thus, contribute to the progress of research in bone diseases.
In parallel to this project, I will also be conducting a systematic review of published clinical trial studies in cell-based immunotherapies under the supervision of David Brindley, Oxford Academic Lead and Director of Research Programmes. Here, I endeavor to evaluate the efficacy of studies encompassing therapies derived from the manipulation of immune cells that have differentiated from their lymphoid progenitors residing in the bone marrow and thymus. The progeny cells that arise from the lymphoid parents are called T lymphocytes, natural killer cells, dendritic cells, and monocytes/macrophages. These cell types are used in applying their pathogen recognition abilities to patients suffering from pathogenesis. For example, immunotherapies are being used to battle cancer. Here, a receptor on a T lymphocyte that is used in detection and binding of their target can be genetically modified to detect and bind a cancer cell. The product is a disabled cancer cell that is targeted for destruction.
Currently, a systematic review of this topic is novel. I plan to stratify the individual cell-based immunotherapy studies by various factors and use the accumulated data as a tool for the healthcare sector, academia, and cell therapy industry to use in identifying prospective therapeutics. I believe the information I am gathering will be helpful for scientists and clinicians working with cellular immunotherapies by providing a collective history narrating the successes of these medicines.
Future Plans:
I feel my past and ongoing research experiences to be supportive in building my proficiency in a variety of biological and technical research skills. Materializing my creativity through experiments and answering a biological question is what I find exciting. Biomedical researchers have the ability to contribute their findings to human health issues in order to reach a conclusion. They can also redefine these issues to offer a clearer understanding of a disease. It is such a fascinating career, and I am immensely grateful I can contribute to the field. I look forward to building my scientific expertise and applying my growing knowledge to formulate beneficial biomedical solutions. The future path I take will definitely be one involved in researching biomedical treatments for diseases that continue to plague our population.
References:
Jiang Y, Oliver P, Davies KE, and Platt N. (2006) Identification and Characterization of Murine SCARA5, a Novel Class A Scavenger Receptor That is Expressed in Populations of Epithelial Cells. The Journal of Biological Chemistry, 281, 11834-11845.
Kular J, Tickner J, Chim SM, and Xu, J. (2012) An overview of the regulation of bone remodeling at the cellular level. Clinical biochemistry, 45(12), pp.863-873.
Lin YL, de Villiers WJ, Garvy B, Post SR, Nagy TR, Safadi FF, Faugere MC, Wang G, Malluche HH, and Williams, JP. (2007) The Effect of Class A Scavenger Receptor Deficiency in Bone. The Journal of Biological Chemistry, 282, 4653-4660.


