
Short summary: How SENS “damage-repair” longevity therapeutics can prevent and reverse the second most common neurodegenerative disease: neurodegenerative aging of the Parkinson’s type.
Even more than cancer or heart disease, the degeneration of one’s brain is for most people the most feared of aging processes — and the standout stalker of our nightmares is neurodegenerative aging of the Alzheimer’s type (AD).
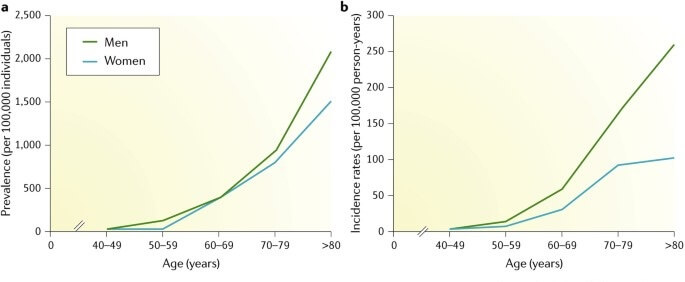
Unless someone close to them has suffered with it, fewer people think immediately of neurodegenerative aging of the Parkinson’s type (Parkinson’s). My guess is that this is in part because Parkinson’s is less common than AD, and in part because the mind of a person with Parkinson’s typically remains intact in the early stages of the disease, even as the body becomes increasingly disabled. But Parkinson’s is a ruinous plague on one’s life, and it does often end in forms of dementia. It’s also more common than has traditionally been thought and is on the rise, over and above what you’d expect from the aging of the population overall.
RepleniSENS to Replace a Critical Set of Neurons
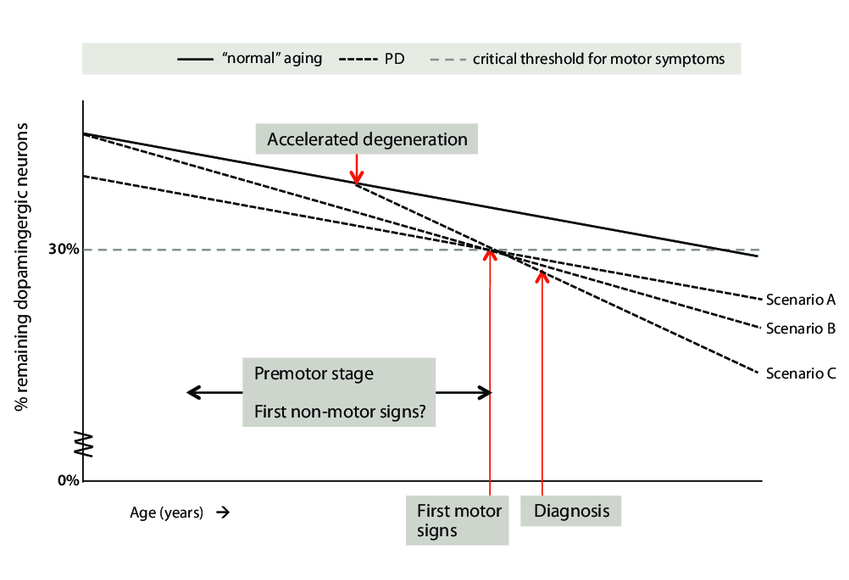
The most visible symptoms of Parkinson’s, and the ones on whose basis people are diagnosed with the “disease,” are what are called the “motor symptoms.” These include trembling hands, a shaking head, difficulty keeping your balance, and a “mask-like” facial expression that blinds the outside world to your emotions. These symptoms result from the progressive loss of — and damage to — a specific population of neurons located in an area of the brain called the substantia nigra pars compacta (SNc). These neurons produce the chemical messenger dopamine, and they use it to fine-tune the signals by which we initiate and control movement by regulating the activity of neurons in another region of the brain.
There is enough built-in redundancy in the SNc that we continue losing these “dopaminergic” neurons for decades without any obvious problems. But once our supply of these neurons dwindles to beneath the “threshold of pathology,” we can no longer make these fine adjustments to the movement-control signals, and the motor symptoms of Parkinson’s subvert the movement of our faces, hands, and bodies.
Almost all the drugs used to treat Parkinson’s work by manipulating dopamine levels or signaling somehow — supplying more of its chemical building blocks, reducing its clearance from the gaps between the neurons, making sure that its precursor isn’t broken down before it reaches the brain, or stepping in biochemically for dopamine by stimulating the same receptor to which it binds. But all the while, a person with Parkinson’s is still losing the key neurons that make and use this dopamine, and simply dumping more of it into the brain in an unregulated way is no substitute for the precisely-orchestrated delivery of dopamine to just the right neurons at just the right time, as the dopamine-producing neurons do so masterfully.
The rejuvenation biotechnology solution to this problem is to repair the damage by replacing the lost neurons. The good news is that scientists have been working on dopaminergic neuron transplantation for longer than any other kind of true cell replacement (RepleniSENS) therapy. Early human clinical trials in the 1980s that used cells derived from aborted fetuses yielded mixed results — but a small percentage of the patients who received the cells experienced remarkable improvements, going off of their medications for years and keeping a few of the transplanted neurons alive in their brains and producing dopamine up to 24 years later.
Because of the politics of abortion, this promising research was cut off from funding by the largest source of biomedical research funding in the world: the US National Institutes of Health (NIH). And the fetal cell therapy procedures were too unreliable and risky to move directly into regular human use. Nonetheless, the fact that some of the patients who got these early transplants improved so much for so long encouraged other researchers to spend the next several decades working out the cell types, transplant sites, and other details to optimize the procedure.
And then came cellular reprogramming technology, the Nobel Prize-winning rejuvenation biotechnology whereby scientists can take a tissue sample from an adult, apply genes or other technologies that activate or produce proteins that turn specific genes on or off, and wind back the developmental clock on a cell. Just as embryos begin their existence as balls of undifferentiated stem cells that eventually develop into liver and intestinal cells, brain neurons, muscle cells, and all the other cells of the body as they develop, so too these “induced pluripotent stem cells” (iPSCs) have wide-open developmental potential. Scientists can then apply different cues to nudge a reprogrammed cell into becoming the kind of cell they need for therapeutic or scientific purposes.
Cellular reprogramming offers two strong advantages over embryonic stem cells. First, it allows doctors to engineer youthful therapeutic cells without the funding restrictions and pseudo-ethical debates that surround fetal or embryonic stem cells. And second, because doctors can take the initial cell biopsy from the same person who needs therapeutic cells, cells derived from reprogramming are treated as “self” by the patient’s immune system and do not require immune suppression to prevent rejection.
After years of work, including the remarkable first transplantation of skin cells reprogrammed into dopaminergic neurons into the brains of nonhuman primates, numerous human clinical trials are now underway to test different reprogrammed cell types to replace lost neurons in people suffering with Parkinson’s disease.
BlueRock Therapeutics, a stem cell company now owned by Bayer (“the aspirin people”), uses proprietary bioprocessing to create stable master cell banks of what they call “universal iPSCs,” which they have found to be compatible with the immune system of any patient. BlueRock scientists then differentiate these cells into dopaminergic neurons for transplant into the brains of people with Parkinson’s. They recently reported positive safety results from a Phase I trial, with evidence of clinical improvement, especially in the people in the group that they gave the largest “dose” of transplanted cells. In the future, they plan trials to do the same thing with new reprogrammed heart muscle cells for people with heart failure and other diseases of aging and beyond.
Aspen Neuroscience — founded and scientifically led by SENS Research Foundation Research Advisory Board member Jeanne Loring — is working with reprogrammed cells taken from the same people with Parkinson’s that they intend to treat with them, after differentiating them into dopaminergic neuronal precursor cells that they call ANPD001. They recently reported efficacy in an animal model using cells derived from several different human Parkinson’s patients, were granted fast-track status by FDA, got approved to begin clinical trials, and are gearing up for a Phase I/IIa, single-arm, open-label dose-escalation trial.
Meanwhile, a group of Korean scientists recently reported the results of proof-of-concept animal studies using their human embryonic stem cell-derived dopaminergic neurons. When they implanted these neurons into the brains of a rat model of Parkinson’s, the graft took, the neurons extended to their proper target, and the motion disorder they had created in the rats to model Parkinson’s progressively faded away. The researchers (or their biotech company) have established a biological production plant where they can produce these neurons on a large scale at a quality suitable for human clinical use. Their biotech company is now organizing a Phase I/IIa human clinical trial of these cells in people with Parkinson’s.
But the most dramatic scientific story of scientists implanting reprogrammed dopaminergic neurons into people with Parkinson’s is the self-funded experiment by biomedical device entrepreneur George “Doc” Lopez. Less than a decade into Lopez’sParkinson’s diagnosis, the standard dopamine-boosting Parkinson’s drugs were starting to fail him — and as a doctor, he knew all too well the accelerating decline he was facing. When his neurologist told him that the best prospect for a cure lay with stem cells, he hopped a flight to a scientific conference on the subject.
There, he was gripped by a presentation by stem cell researcher Kwang-Soo Kim of the Harvard-affiliated Maclean Hospital, who presented his work on reprogramming human patient cells into midbrain dopaminergic neurons using a version of the reprogramming factors that all but eliminates any risk of mutations. After further discussions with Dr. Kim, Doc Lopez cut Kim’s institution a cheque for $2 million to scale the process up so they could make enough cells for human clinical use. Lopez then recruited neurosurgeon Jeffrey Schweitzer to do the transplants. The team then spent years doing scientific work and running dry runs of the complex multi-location protocol that required them to derive and validate the neurons and then transplant them into a human patient during a window of a few short hours.
Finally, Schweitzer was ready to implant the iPSC-derived neuronal precursors into Lopez’s brain. The researchers reported in 2020 that they produced reprogrammed cells from Lopez’s deep skin cells under Good Manufacturing Practice (GMP) conditions for human clinical use. The cells have the molecular signatures and functional behavior of dopaminergic neurons. And importantly, they don’t set off an immune reaction when scientists implant them into mice whose immune systems were “humanized” with Lopez’s own blood cells, giving them confidence that patients’ systems will not attack the transplanted cells. Accordingly, Mr. Lopez’s doctors did not prescribe any immunosuppression for the transplant.
The surgery proceeded without incident. After that, Lopez went in for periodic PET scans and clinical testing to see if the cells had “taken” and how they affected his symptoms. Labeled PET tracers that light up when dopaminergic neurons are active showed a concerning dip in activity at the three-month mark, but after that, there was a modest increase in activity at the one and two-year mark, implying that the neurons had survived and were functional — particularly on the right side. (Remember, the default trajectory if Lopez had not received the implants would have been an accelerating loss of these cells). And at clinical testing sessions 18 months and two years after surgery, Lopez’ Parkinson’s symptoms remained stable instead of undergoing the expected end-stage decline.
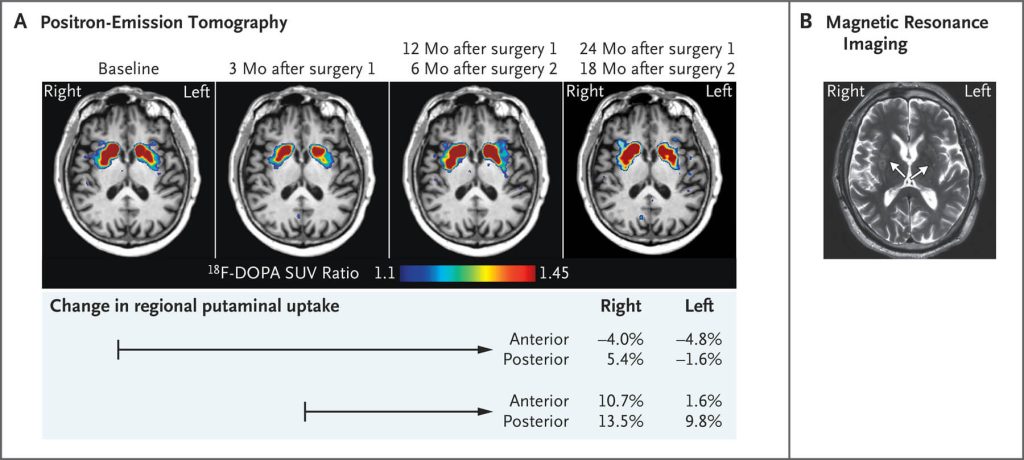
The researchers reported the results of this dramatic race to move an innovative rejuvenation biotechnology from the lab bench into Mr. Lopez’s brain in the spring of 2020. But it got little attention at the time, because the world had something else on its mind: the COVID pandemic. Public health measures also forced the scientific and clinical team to shelve their plans for a full-on clinical trial, but Dr. Schweitzer assures me that they are back on track and will be underway soon.
Smaller players in the cell replacement space include Dr. Jun Takahashi of Kyoto University in Japan, whose ongoing trial recruited just seven subjects and administered cells to its first patient in November of 2018, but hasn’t reported anything since. And we’re even still learning things about grafting fetal neurons, because of a revamped European trial with curtailed ambitions known as Transeuro.
LysoSENS and AmyloSENS to Clear Alpha-Synuclein Aggregates
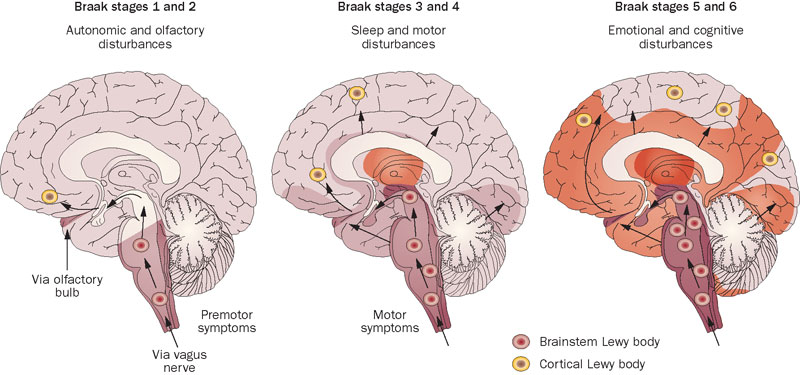
Our brains accumulate aggregates comprised of the protein alpha-synuclein (AS) as we age, both inside and between our neurons. People suffering from diagnosed Parkinson’s and closely-related neurological aging disorders bear especially high burdens of these aggregateas. The various forms of aggregated AS first appear in neurons outside of the brain (particularly the gut) and later invade the brain, starting from the base of the skull and then insidiously spreading forward across the brain over the course of aging and disease.
AS aggregates are closely linked to the “non-motor symptoms” (NMS) of Parkinson’s, which manifest earlier in the disease than the motor symptoms but usually do not lead to a PD diagnosis. Doctors and patients usually don’t pick up on NMS in part because the NMS are initially fairly mild, and in part because many NMS are the kinds of vague, general nuisances that people often write off as “just aging” or blame on any number of other causes. Such early-arising NMS include constipation and other GI symptoms, loss of one’s sense of smell, incontinence, and erectile dysfunction. Anxiety or depression can also be unrecognized results of the early stages of Parkinson’s.
REM sleep behavior disorder (RBD) is the one NMS that acts as a strong red flag for Parkinson’s. In RBD, the brain of a person who is on the path to PD fails to turn off movement during their dreams, which results in them starting to act out their dreams in the real world — sometimes violently or upsettingly. As the disease progresses, NMS become more and more severe, and can ultimately include dementia, hallucinations, and obsessive, stereotyped, repetitive behaviors called punding. There are no specific therapies for the NMS: clinicians improvise, treating them with drugs and treatments that were originally studied to treat similar symptoms in a non-Parkinson’s context.
Fortunately, researchers are currently running clinical trials to test numerous AmyloSENS therapies to clear AS aggregates located outside of cells. (Most of these trials are in Phase II, meaning that they are beyond basic safety testing but are not yet designed to clearly establish whether something is effective or not or lead to approval by regulators like the FDA). Some of these rejuvenation biotechnologies seem to be working in preliminary clinical trials: for instance, we covered the first human clinical trial of an AmyloSENS vaccine therapy targeting alpha-synuclein (PD01A) and its subsequent progress, and new clinical trials have begun on an optimized formulation of PD01A called ACI-7104.056.
Unfortunately, no one has yet developed LysoSENS therapies to target AS aggregates inside the cell, and it’s these intracellular AS aggregates that likely inflict the greatest harm. The main reason for this seemingly backward prioritization is that it’s not obvious how you would target AS aggregates inside cells. The go-to approach of using therapeutic antibodies to capture disease-driving aggregates doesn’t seem likely to work, as antibodies typically do not enter cells — or if they do, they are often sealed away from the contents of the cell and can’t do anything once they get there.
Nor can we use the original LysoSENS strategy of engineering enzymes to digest AS aggregates inside the lysosome, because AS aggregates don’t build up in the lysosome! Instead, they accumulate in the main body of the cell. And whereas the lysosome seals its contents away from the rest of the cell so that it can safely attack stubborn biological wastes, it wouldn’t be safe (or likely feasible) to send in powerful enzymes to attack these aggregates outside the lysosome because they might start tearing into important biological machines or interfering with necessary biochemical reactions.
Fortunately, there is a potential path forward. Several years ago, Dr. Aubrey de Grey noted a report in the scientific literature on a novel way to smuggle antibodies into cells intact. That trick alone wouldn’t be enough to solve the problem, because even if you got excellent antibodies into the cell and they bound to AS aggregates, how would you get the bound antibody-aggregate complex out of the cell again — let alone remove it from the brain altogether?
But he realized that instead of using the antibody-smuggling technology to deliver conventional binding antibodies into the cell, scientists could instead send in catalytic antibodies (catabodies) to chop pathological aggregates into tiny pieces — and they would do it right there in the main body of the cell, eliminating the need to get it out of the cell afterward. Moreover, whereas conventional binding antibodies can only latch onto a single aggregate and then have to somehow drag it away from the tissue it’s afflicting, catabodies can attack one aggregate, macerate it into manageable fragments, and then go on to attack the next aggregate — and the next, and the next.
SENS Research Foundation scientists worked for the last several years to develop this intracellular aggregate-targeting catabody approach. Their first target is tau oligomers, which are the kind of abnormal tau whose accumulation inside aging neurons is most likely to drive neurodegenerative aging of the Alzheimer’s type, as well as broader age-related cognitive decline. An initial set of starting antibodies proved to be unusable. They have developed a new plan to advance the project in the future once new funding is available. If the intraneuronal catabody approach works against tau oligomers as we expect it to do, it could become a platform technology that we could then apply to target AS aggregates, leading to their elimination on-site inside neurons.
MitoSENS for the Hotbed of Mitochondrial DNA Deletions
As we age, a small percentage of long-lived cells that don’t divide (such as neurons and muscle cells) get completely taken over by mitochondria that have suffered the loss of huge chunks of their DNA. And of all the cells in the body, the cell type that is most susceptible to this hostile takeover is the critical population of dopaminergic neurons whose loss is central to Parkinson’s. Scientists think one reason for this high vulnerability is that these neurons are much more active than other kinds of neuron (including dopaminergic neurons elsewhere in the body), which causes them to produce more free radicals as a consequence of greater energy production. Another reason relates to the way that SNc dopaminergic neurons handle calcium fluxes. Smaller DNA deletions may also contribute to Parkinson’s , even if they don’t completely overtake the cell.
It’s not clear what the connection is between these DNA deletion-bearing mitochondria and the loss of dopaminergic neurons with age, but it seems safe to assume that even if they don’t kill their host neurons, deletion-bearing mitochondria sweeping across the cell leads to an energetic brownout that makes any surviving neurons less effective at delivering a regulated pulse of dopamine to their target cells. Additionally, large mitochondrial DNA deletions impair the ability of cells to release substances they produce for export, which would make afflicted neurons unable to release even the dopamine they do manage to produce.
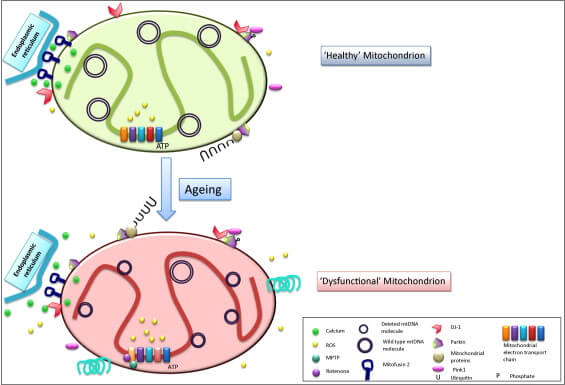
The MitoSENS lab at SENS Research Foundation is working to develop three different platform technologies (including the original MitoSENS strategy of allotopic expression) to prevent, replace, or bypass mitochondrial DNA mutations. Rehabilitating the mitochondria in dopaminergic neurons is one of the strongest use cases of this core rejuvenation biotechnology.
ApoptoSENS for When “The Butler Did It”
All Together Soon
As is the case with many particular, diagnosable diseases of aging, the scientists working on these various “damage-repair” therapies are largely operating in silos. Some of them are working to develop a standalone rejuvenation biotechnology for Parkinson’s; this is the case for RepleniSENS therapies to replace dopaminergic neurons and LysoSENS and AmyloSENS therapies to clear out alpha-synuclein aggregates. Other scientists are targeting cellular and molecular damage that drives Parkinson’s, but their path to human clinical use runs through an unrelated disease of aging (such as UNITY Biotechnology’s ApoptoSENS therapy for diabetic macular edema) or even diseases entirely unrelated to aging (such as most MitoSENS work, which will likely first be licensed for congenital mitochondrial mutations that afflict young children).
All of these therapies will eventually need to be tested together in combination trials to determine the best regimens to use them together to maximize efficacy and minimize side-effects. We’ve already seen a lot of evidence that AmyloSENS therapies to clear beta-amyloid are much more effective when delivered before too much aberrant tau accumulates in the brain, which reciprocally implies that LysoSENS therapies to clear aberrant tau would greatly enhance the effectiveness of beta-amyloid clearance. But for now, we can celebrate the progress being made in each of these domains individually, and dream of the day when our hands will stay steady indefinitely, and our heads only shake to say “no” to aging.



