
How Might Cyclarity’s UDP-003 Compare to EDTA Chelation?
SENSible Question: Can you contrast the potential effect of SRF spinout Cyclarity Therapeutics’ UDP-003 versus EDTA on reducing CAD?
This question is asking for an apples-to-oranges comparison: EDTA chelation and UDP-003 are not merely different molecules that target atherosclerotic cardiovascular disease (ASCVD) in different ways, but their potential effects are quite different, as is the evidence supporting them.
Let’s start with EDTA chelation. EDTA is a chelating agent, meaning that it can bind tightly and form a soluble compound with various metals. Chemists, biologists, and the food and beverage industry have found multiple uses for EDTA, from blood banking to cell separation to food preservation — and it’s also FDA-approved for treating lead poisoning, which is where the idea of using it for patients with atherosclerosis began. In the early 1950s, doctors treating patients for industrial lead poisoning with EDTA noticed that some of their patients with existing ASCVD would report relief from chest pains upon exertion (stable angina). That made doctors wonder if maybe EDTA was somehow regressing their atherosclerotic plaques.
Cardiology was in its infancy at the time, and we didn’t really understand what atherosclerotic plaques were or what caused them. But doctors knew that some advanced plaques contained calcium deposits, and other specialists had reported that EDTA could lower the burden of so-called “metastatic calcification,” which is abnormal calcium deposits that form in organs when blood calcium and/or phosphate levels are high for any of several reasons. So some doctors wondered if EDTA was similarly removing calcium from their lead poisoning patients’ atherosclerotic lesions, allowing blood to flow more freely into their hearts and thus relieving their stable angina.
There are a few problems with this hypothesis right out of the gate. First, no one has ever convincingly shown that intravenous EDTA actually removes calcium from atherosclerotic plaque, and it’s not clear that EDTA can even physically reach plaque calcium. Calcium levels in the blood do rise shortly after treatment, and urinary calcium excretion increases, but it’s more plausible that EDTA is drawing this calcium out of patients’ bones than that it is extracting it from their plaques — or even that it would come disproportionately from their plaques, which contain a tiny fraction of the overall calcium content of the body.
Second, if somehow EDTA chelation could remove calcium from calcified plaques, there isn’t enough calcium as a percentage of the lesion volume to increase blood flow to the heart and thus explain the reported improvement in stable angina symptoms. In fact, coronary calcium burden doesn’t correlate with the amount of blood flow interference to the heart caused by atherosclerosis that is responsible for stable angina pain.*
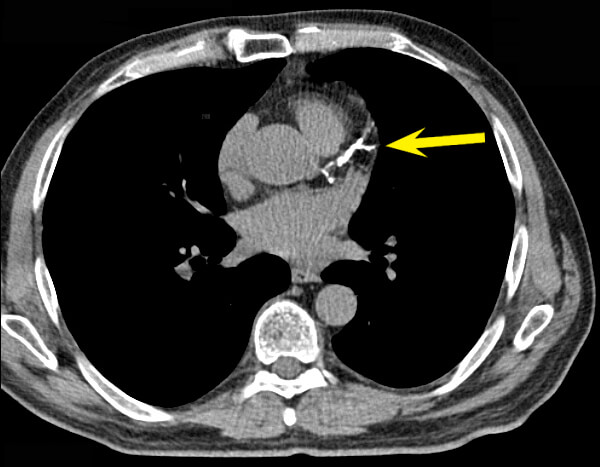
And third, while it might have sounded like a good idea to remove calcium from atherosclerotic plaques back in the 1950s, seven decades of subsequent research cast this idea in serious doubt. It’s now clear that the bulk of the calcium deposits in atherosclerotic plaque — the part that you readily see on a coronary calcium scan — don’t coincide with the lesions that go on to rupture and trigger heart attacks and strokes. The value of the coronary calcium scan is not that it reveals the specific atherosclerotic lesions that are likely to kill you, but that the overall extent of calcified plaque gives a convenient index of a person’s total burden of advanced lesions, and thus the risk to the patient. From this comes the saying, “Coronary calcification identifies the vulnerable patient rather than the vulnerable plaque.”
None of this directly tells us whether EDTA chelation is any use in preventing or treating ASCVD: for that, we need clinical trials. Clinicians have run a number of such trials, mostly of low quality. They have also performed a meta-analysis that pooled together the evidence from all of the aforementioned trials, and could find no clear evidence of benefit, although the designs of the studies were so different that it was hard to combine them convincingly.
So there are a lot of reasons to be skeptical of EDTA therapy for ASCVD. Yet the largest of the EDTA trials, called the Trial to Assess Chelation Therapy (TACT), hints that it may actually benefit some people — just not as much as or in the way that chelation doctors initially thought that it would.
In the TACT trial, 1708 patients over the age of 50 who had suffered a heart attack began were randomly assigned to receive 40 weekly infusions of either an EDTA cocktail of the sort usually used by doctors who provide EDTA therapy or a saline placebo infusion. On top of whichever infusion they got, each patient also received either a multivitamin (which EDTA doctors often prescribe to counteract potential mineral deficiencies resulting from EDTA treatment) or a placebo pill. The trial doctors would look to see if patients receiving EDTA instead of placebo infusions would suffer less of a “composite outcome” comprised of several different major cardiovascular catastrophes combined: heart attack, stroke, surgery to restore blood flow to the heart (like stents and angioplasty), hospitalization for unstable angina (which is a dangerous event, not just the chest pains of stable angina), and death overall.
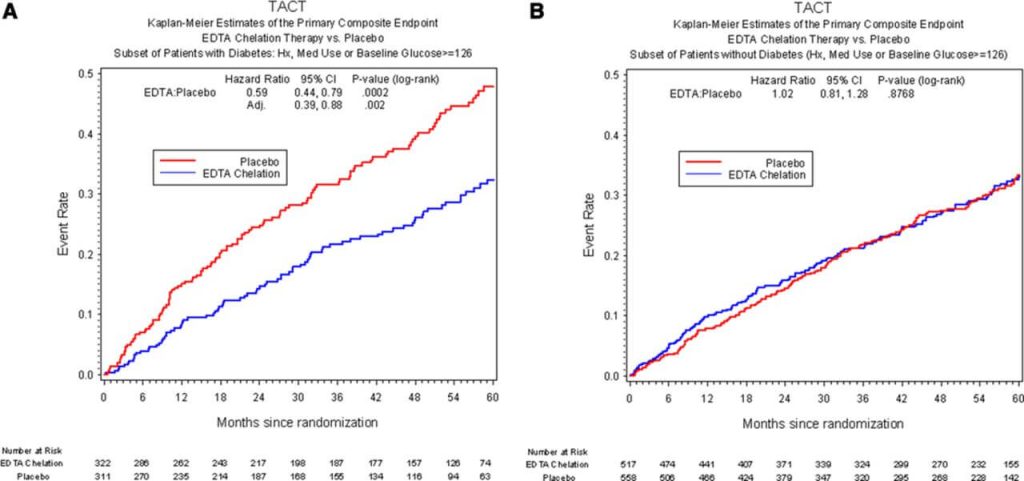
To the study investigators’ own surprise, it seemed to work! Overall, there was about an 18% lower rate of the composite outcome in EDTA-treated patients than in people receiving saline infusions, with the single clearest contribution coming from patients needing fewer surgical blood flow restoration procedures.
Things became even more interesting when the investigators did a second analysis that they had always planned to do, in which they looked separately at the effect of EDTA versus placebo in patients who did suffer from diabetes and those without it. In this analysis, the effect of EDTA was suddenly split in two. On the one hand, diabetic patients who got EDTA treatment after a heart attack suffered a remarkable 41% fewer “composite outcome” cardiovascular crises. In fact, the reduced rate of cardiovascular events in people with diabetes was so large that it accounted for all or nearly all of the difference EDTA appeared to make in the primary analysis of the trial. In other words, EDTA appears to have been very protective in people with diabetes who had suffered a heart attack, but offered no protection at all in patients who did not have diabetes.
So why might EDTA benefit people with diabetes even though it doesn’t help most high-risk ASCVD patients? Before they launched the trial, the TACT investigators had hypothesized that it might turn out that way, based on the additional risk that comes with the metabolic state of diabetics. There’s good evidence that two metals other than calcium that EDTA strongly draws out of the body (cadmium and lead) accelerate the course of cardiovascular disease. The metabolic derangements of diabetes put people with the disease at much higher risk of ASCVD, heart attacks, and strokes than other people who otherwise have the same cardiovascular risk factors, and those abnormalities could plausibly interact with the damaging effects of these two metals. So lead and cadmium exposure might be especially harmful to people with diabetes, such that using EDTA to draw those metals out of the body might benefit people with diabetes even if it fails to help people without the disease.
Because the investigators hadn’t strictly designed TACT to test this idea, and because there hasn’t been clear evidence of such an effect in other trials, we can’t point to TACT as having proven that EDTA is beneficial in people with diabetes who have suffered a heart attack. But we should have a clearer idea soon. The successor trial TACT2 is underway to see — hopefully once and for all — if diabetic heart attack patients really do suffer fewer cardiovascular calamities if treated with IV EDTA with or without a multivitamin.
A Hypothetical Heart Head-to-Head
So how does EDTA stack up against Cyclarity’s candidate therapy UDP-003? For the sake of thinking about that question, let’s put aside the otherwise critical question of whether EDTA really reduces the risk of cardiovascular events and pretend we know it works as advertised. Let’s even set aside the fact that if intravenous EDTA does benefit patients with cardiovascular disease, it’s apparently only effective in the subset of them that is diabetic. Instead, let’s focus on what the two therapies are supposed to be doing about the problem.
Having seen the flaws in the calcium hypothesis, the TACT investigators are focusing on the chelation of toxic metals, which seems to fit with the apparent benefit in diabetic patients. The evidence linking a high burden of these metals to cardiovascular disease is substantial — and it will get even more convincing if TACT2 is successful. So to compare what EDTA appears to be doing in heart attack survivors to what Cyclarity’s scientists expect UDP-003 to do, it’s essential to understand why lead and cadmium would jack up a person’s risk of cardiovascular events.
To summarize decades of epidemiology, animal studies, and molecular biology, these toxic metals exacerbate plaque formation and instability by sparking more radical formation and inflammation; by interfering with the ability of the cells lining the blood vessels to relax and allow blood to flow freely (in part but not exclusively through free radicals and inflammation); and possibly by disrupting the tight barriers between the cells of the artery wall.
All of those things would increase the risk of a heart attack in people with ASCVD, either by making blood clots more likely, or by recruiting more immune cells into the lesions, or by impeding the flow of blood into the heart in a crisis. So in principle, it would make sense if removing them from the blood with EDTA would reduce the risk of a heart attack — even if the effect was subtle enough that it would only move the needle in people with the background metabolic abnormalities of diabetes.
But none of these mechanisms get at the root cause of ASCVD: macrophages swallowing one too many of the LDL particles that have infiltrated into the artery wall, leading to the failure of their lysosomes to process the cholesterol in the particle. Paralyzed by the overload of cholesterol and its oxidized byproducts, the macrophage lays down connective molecules and becomes an immobilized foam cell in the artery wall.
Foam cells accumulating in the artery wall are the key players in the whole atherosclerotic process. They inflame the tissue; they attract more immune cells into the lesion; and eventually, they die a messy necrotic death. This recruits yet other macrophages to clean up the toxic foam cell remnants, but over time the increasingly-desperate cleanup crew also starts to suffer losses as they are poisoned by the same toxic cholesterol products that originally felled the foam cells, exacerbating the lesion.
The artery attempts to seal up this mass grave of dead and dying macrophages and foam cells under a “fibrous cap,” but the lesions are unstable and prone to rupturing. And when the lesion cap finally does fail, the deathly goo that spews out of the plaque triggers blood clots that hurtle down to the small vessels feeding the heart or the brain, like gooey torpedoes aimed at these critical organs — armed to trigger a heart attack or stroke.

Taking Atherosclerosis Out at the Root
Cyclarity’s approach is radically different from EDTA. Based on the toxic metal hypothesis, removing lead and cadmium from atherosclerotic lesions would be expected to slow the spread and exacerbation of atherosclerotic plaques and cool the fires of inflammation and oxidative stress in and around them. The results of TACT appear to tell us that EDTA therapy can do just that in diabetics after a heart attack; TACT2 will either confirm that idea or leave us with more questions.
By contrast, Cyclarity’s UDP-003 is designed to be nothing less than a small molecule LysoSENS therapy that directly removes some of the damaged cholesterol products that turn macrophages into foam cells and thus drive the core of ASCVD. If it works as expected based on studies to date, Cyclarity’s novel cyclodextrin will not just turn off the fan driving the flames of ASCVD, but will actually prevent and reverse atherosclerosis itself.
UDP-003 is a custom-built form of cyclodextrin — a complex barrel-shaped carbohydrate that can capture and securely lock away other molecules. The path to UPD-003 began with hydroxypropyl-beta-cyclodextrin (HBCD), a common cyclodextrin used in the food, cosmetics, and drug industries. In the 1990s, scientists showed that HBCD could capture not only cholesterol itself, but an especially toxic damaged form of cholesterol known as 7-ketocholesterol (7KC). And having captured it, HBCD could by its own action draw 7KC it out of foam cells in a dish.
7-ketocholesterol is a bad actor in aging: it appears to be a major villain in the dysfunction and death of the related cells in the eye whose downfall drives age-related macular degeneration (ARMD — the leading cause of blindness in people over the age of 65). More to our immediate point, 7KC is also poisonous to macrophages, and thought to be a key culprit in turning them into foam cells. In addition to 7KC being generated when the cholesterol carried by LDL particles is oxidized while mired in the complex structures of the inner artery wall, the macrophages themselves may unintentionally generate some 7KC during the processing of cholesterol from engulfed LDL particles, possibly because their lysosomes contain reactive iron left over from degrading iron-containing proteins and organelles.
The major takeaway from all this is that removing 7KC from macrophages and other vulnerable cells offers a potential rejuvenation biotechnology to prevent and reverse ASCVD at its root, by maintaining and restoring the ability of arterial macrophages to process the cholesterol in LDL particles. Instead of seizing up under the burden of too much total and oxidized cholesterol and degenerating into foam cells bogged down in the very tissue they went in to rescue, arterial macrophages would continue protecting the arteries from LDL particles enmeshed in their inner walls, and then leave harmlessly when their job was done. Keeping macrophages clear of 7KC would also allow the secondary macrophages that enter the lesion to clear out dying foam cells to complete their work, speeding the regression of even established plaques.
Years after the first observation that HBCD could remove cholesterol and 7KC from foam cells, scientists showed that this same common cyclodextrin could potentially treat children with Niemann-Pick C1 disease (NPC), a terrible congenital disorder that prevents them from moving cholesterol out of their cells. And in mice, treatment with this cyclodextrin was able to reverse atherosclerosis in a mouse model of the disease.
So why aren’t we all mainlining HBCD? The main problem is that HBCD is a fairly sloppy molecule: it’s too prone to draw normal cholesterol out of the cell along with 7KC. That might seem fine: we’re used to thinking of cholesterol as a villain. But the fact is that cholesterol is an absolutely necessary molecule in our bodies: it’s essential for the normal structure and functioning of cell membranes, and it’s also the raw material from which our bodies synthesize a wide range of essential biomolecules, including vitamin D, sex hormones, and the stress-adaptation hormone cortisol.
The fact that HBCD will suck unmodified cholesterol out of any structure of the body results in some critical side effects that limit its use even in children whose NPC leads to severe neurological disease. Even at the doses currently used, most children treated for neurological complications of NPC with HBCD will develop some amount of hearing loss due to damage to the nerve cells in the inner ear.
A Molecular Precision Pincer
If you could somehow smuggle cholesterol exclusively out of the lysosomes of foam cells and leave all the other cholesterol in the body untouched, that would be one thing — but that’s asking for magic, not pharmacology. Instead, the then-SRF scientists Drs. Matthew (“Oki”) O’Connor, Daniel Clemens, and computer scientist Amelia Anderson— who would go on to found Cyclarity with then-SRF CEO Mike Kope — set out to design a molecular precision tool that would remove 7KC selectively while leaving normal physiological cholesterol untouched. Using a combination of experimentation with existing cyclodextrins, trial-and-error virtual experiments in existing molecular modeling software, and eventually a new VR molecular modeling and design system, Cyclarity constructed a novel cyclodextrin dimer in virtual space and tested it in silico against native and oxidized cholesterol before testing it in actual lab conditions — and then back again to the virtual system in an iterative optimization cycle.
This dimer is comprised of two different cyclodextrins, one of them “scoop-like” and the other a “gripper” complex, linked together in a configuration where the two can cooperate to create a binding cavity. It’s a bit like a reusable coffee pod or a shaker cup for protein shakes, with a chamber to take in the desired material and then a sealable cap to hold the contents in place. This dual structure allows UDP-003 to first capture 7KC and then surround it, holding on to it with extremely high affinity. Trapped in the UDP-003 core, 7KC can’t interact or react with anything else in the body, allowing UDP-003 to passively carry it out of the cell, through the circulation, and harmlessly out of the body via excretion.
In experimental systems, UDP-003 sucks very high amounts of 7KC from cultured cells and blood cells, as well as from surgically captured atherosclerotic plaque, with no corresponding effect on free unmodified cholesterol in blood. A useful safety test is to see if a cyclodextrin will extract cholesterol out of the membranes of red blood cells, causing them to lyse; on such tests, UDP-003 has extremely little hemolytic activity.

Showtime for Two Small Molecules
Both IV EDTA and Cyclarity’s UDP-003 are approaching a crossroads. IV EDTA has been used in humans for 70 years without ever actually knowing if it does anything; with TACT2 well underway, we should know soon if it really can prevent a basket of bad cardiovascular outcomes for people with diabetes.
By contrast, UDP-003 has never been inside the human body, unless you count human cells in a dish — but that’s about to change. In 2021, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA — their equivalent of FDA) awarded Cyclarity an Innovation Passport under its Innovative Licensing and Access Pathway (ILAP), which is a new program designed to help shepherd truly innovative therapies more quickly through the regulatory process by giving awardees early and ongoing contact and feedback with the regulators.
Thanks to this and a solid scientific foundation, UDP-003 trials are coming up fast in the UK. As of this writing (late winter of 2023), Cyclarity is finishing up its animal safety data and getting ready to produce enough UPD-003 at pharmaceutical grade to run their first human trial. While only a safety trial, it will be a critical first-in-human test of the new molecule. And if UDP-003 is as powerful as the Cyclarity team anticipates and the followon trials are well-structured, we could know if Cyclarity’s novel rejuvenation biotechnology is truly a step-change in prevention and treatment of ASCVD within a few short years.
To Us From Failing Hands They Throw The Torch
The most important question about both of these molecules is what they will actually do in clinical trials. Will people taking them have more vitality? Will they be able to walk uphill without pain in their chests or legs? Will the drugs prevent heart attacks and strokes? Will their side effects be manageable? Will people who take them live longer? We just don’t have these answers yet: that’s what clinical trials are for.
But there’s a crucial difference in what we can expect the two molecules would do if they do work. Periodically lowering the burden of toxic metals in the circulation or even the plaque using EDTA would in principle slow down the rate at which new atherosclerotic plaques form, and tone down the aggravation of existing ones — and it would reduce the risk that vulnerable plaques would rupture and trigger a heart attack. That would undoubtedly be a valuable tool for people with ASCVD: slower progression is better than faster, and even if it did nothing at all about the underlying disease, people with diabetes and ASCVD would suffer fewer heart attacks, strokes, and unstable angina crises. But it would only be a kind of pharmacological safety net, capturing sick people at the periphery as they fell, when what we need is a game-changer.
Cyclarity’s UDP-003 could be that game-changer. Death rates from ASCVD were terrifyingly high in the middle of the 20th century in much of the industrialized world: other than aging itself, the main reason was the widespread rise of smoking, followed by high levels of saturated and later trans-fatty acid consumption. The death toll finally began to fall in the 1970s, initially because later generations were less likely to take up smoking and saturated and trans-fat intake declined and because blood pressure medications became available.
From the 1990s onward, deaths from ASCVD continued to decrease, but instead of public health leading the way, the credit increasingly went to medical science. Pharma companies developed increasingly powerful statins and other lipid-lowering drugs; clinical trial evidence rolled in that encouraged cardiology societies to issue guidelines that progressively expanded the ranks of people advised to take them; and doctors increasingly prescribed them to their patients.
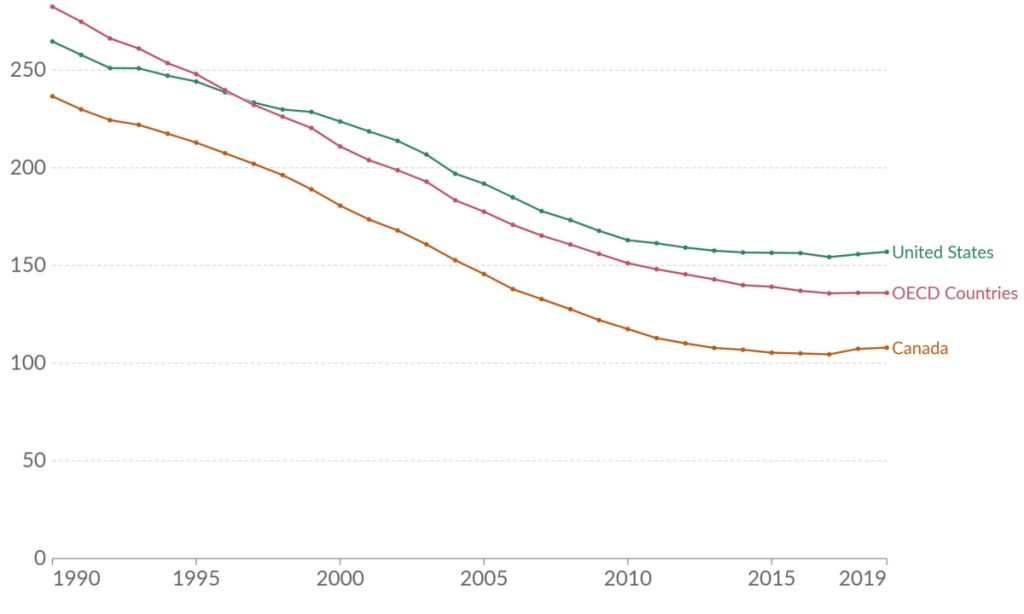
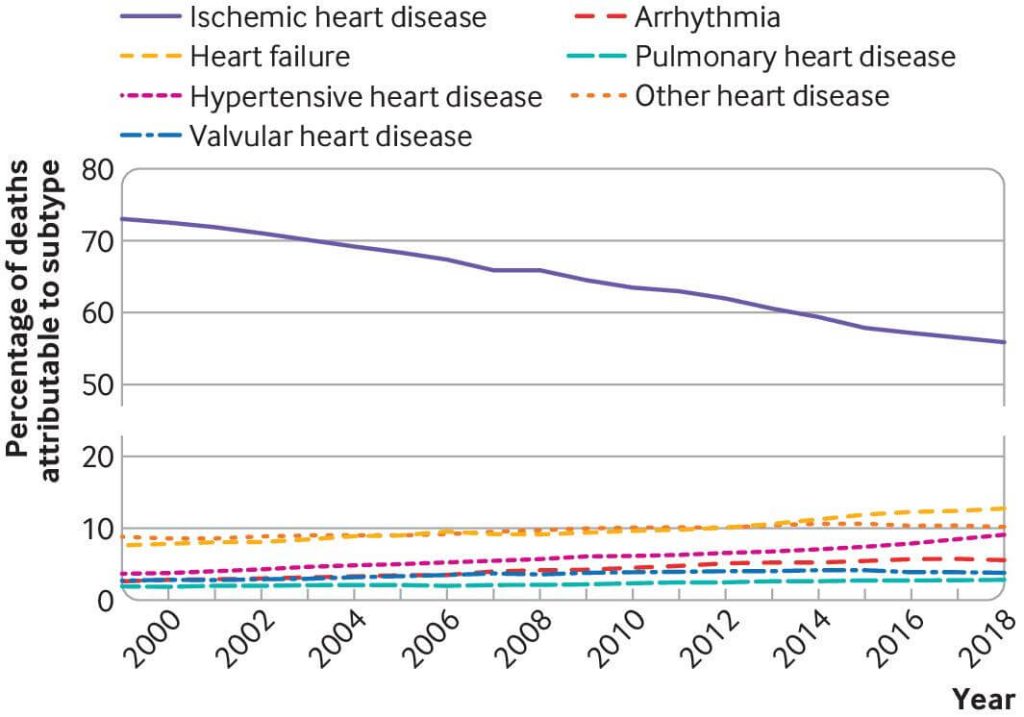
There are still gains to be made and lives to be saved, both by extending existing medicine and public health measures to more people and through new medicines: PCSK-9 inhibitors to dramatically and more safely lower LDL cholesterol; bempedoic acid to do it in the few people who really can’t tolerate statins; and new therapies to lower lipoprotein (a) (Lp(a)) would be a boon to the 20% of us with elevated levels of this lipoprotein, which can be expected to save lives over today’s standard of care in the population overall.
Further lowering LDL cholesterol, blood pressure, blood sugar and insulin, and getting people off of tobacco will surely benefit some patients, but we are reaching the limits of tolerable reductions in the metabolic drivers of ASCVD. Despite their contribution to disease, all of these aspects of metabolism that push us toward heart attacks and strokes are simultaneously essential to our moment-to-moment survival. (The exception is of course tobacco — but even nonsmokers breathing air that is well within current pollution regulations inhale particulate matter and heavy metals that contribute to ASCVD deaths). Blood sugar is essential to life, but just one teaspoon of glucose circulating in our blood separates the level of blood glucose needed to sustain life from a diagnosis of diabetes, and half that amount in the other direction is all the distance to hospitalization from hypoglycemic shock.
Generations of scientists have toiled to bring deaths from ASCVD to a level that is low by the standards of the years after infectious disease ceased being the major cause of death of people in industrialized countries. But to carry forward that torch — to spark another revolution in cardiovascular medicine, and to add to the armamentarium of a comprehensive panel of “damage-repair” therapies needed for longevity escape velocity — we need to go beyond restraining the metabolic drivers of ASCVD.
At the bottom line, atherosclerosis is a disease of aging — that is, of the accumulation of cellular and molecular damage in our tissues. That’s why without knowing anything else about a person — LDL cholesterol level, being a smoker or not, blood pressure, or anything — age (and sex) alone comprise 75% of a person’s risk of cardiovascular disease and major cardiovascular events like heart attacks and strokes. To realize a future free of ASCVD, we will need to strike not at the metabolic pathways driving the damage of aging, but at the damage itself: in the case of ASCVD, the unprocessable cholesterol products that paralyze our macrophages’ lysosomes.
Cyclarity’s UDP-003 (and Repair Biotechnology’s Cholesterol Degrading Platform (CDP), which we discuss in our main LysoSENS page) are aimed squarely at this damage: the time has come for them to prove their potential in human lives.
[*] Note that the calcification associated with advanced atherosclerosis and seen on a coronary calcium scan is only one kind of age-related cardiovascular calcification. While atherosclerotic arterial calcification is a marker of ASCVD severity but does not appear to be harmful in itself, there are other forms of cardiovascular calcification that drive tissue dysfunction, disability, and death.
One is calcific aortic valve disease, in which the“flaps” (leaflets) between the lower pumping chamber of the heart and the main artery leading out of it become stiffer as they thicken, scar, and accumulate calcium. The youthful flexibility of the leaflets is essential to their function, which is to allow blood to squirt out of the heart and into the artery when the heart pumps and then seal shut to keep blood from flowing backward between pumps. Aortic valve calcification stiffens the leafletes, forcing the heart to work harder to pump blood and damage itself in the process. This eventually leads to fainting episodes, chest pain and ultimately heart failure.
The other major form of pathological cardiovascular calcification is medial elastocalcinosis, the progressive mineralization of elastin fibrils beneath the surface of the artery with calcium deposits. Elastin is a stretchy protein that allows our arteries to expand like a flexible balloon when the surge of the pulse travels down the artery, and elastocalcinosis causes the flexible elastin structure to gradually harden. You can read more about this and a potential rejuvenation biotechnology to reverse it on our page on extracellular matrix damage (GlycoSENS).



