
SENSible Question: Is there any established correlation between Senescence-Associated Secretory Phenotype (SASP) or senescent cell burden on one side and the measured hsCRP level or any other routinely measured inflammatory marker in an individual on the other side?
Unfortunately not. Scientists first started asking this question around the time of the first proof-of-concept of the value of destroying senescent cells, and initially hoped it would be a straightforward matter of simply measuring the levels of various SASP factors directly in the blood. While none of the components of the SASP are common blood tests like hsCRP, some commercial blood-testing labs do test for specific proteins that are part of the SASP, including interleukin-6 and tumor necrosis factor alpha (TNF-α).
Somewhat surprisingly, however, things did not turn out to be quite that simple, for reasons that aren’t entirely clear. Perhaps the SASP factors concentrate too locally around senescent cells to be easily picked up in the blood. Or perhaps it relates to the fact that none of the individual proteins and lipid derivatives released from senescent cells as part of the SASP are actually unique to senescent cells. Instead, all of the proteins that make up the witches’ brew of proteins that is the SASP are are repurposed growth factors, protein-degrading enzymes, and above all inflammatory signaling molecules that also produced by non-senescent cells in the body to do things like break down damaged muscle, recruit immune cells, remodel injured tissue, and so on. This might mean that the signal from true SASP factors is swamped out by the fact that those same factors are produced at relatively high levels in an aged person’s body for other reasons, in response to their high burden of aging damage.
And precisely because each individual SASP factor is produced by non-senescent cells for other purposes entirely, no one marker can be used as a reliable index of SASP production: the level of any given factor always reflects a mixture of SASP-related production and production for entirely different reasons. As such, measuring just one or even a few SASP factors in the blood and correlating that to the actual number of senescent cells in your body or the level of SASP they’re producing is likely a fool’s errand from the start.
Scientists can largely bypass all of these problems in animal experiments, as they can simply euthanize the animal, remove the relevant tissue, and test the cells for the expression of the genes for SASP factors or the concentration of SASP proteins in the fluid immediately surrounding the tissue. But of course, that’s not a method we can use for testing the level of SASP or senescent cells in humans. Again, because SASP-constituent proteins are present in tissues for reasons unrelated to senescent cells, scientists must collect data on several SASP factors at once in the same tissue — and compare them to the levels of the same factors in a relevant control animal.
Similar multi-component protocols are also commonly used to measure the burden of senescent cells in aging mice and in mouse models of diseases of aging. Alternatively, scientists can use transgenic animals engineered such that cells expressing one important regulator of senescence light up under light of the right wavelength. Again, we can’t use this test for humans, as we have not been thus engineered.
Trouble for Trials
This is annoying enough when you just want to know about the burden of SASP or senescent cells in your own body out of morbid curiosity. But it becomes a real problem once you start trying to develop “senolytic” drugs intended to destroy senescent cells to rejuvenate the body and prevent or reverse diseases of aging in humans. These technical challenges mean that today’s scientists and startups are working with senolytic therapies that they know work brilliantly in mice, but about whose senolytic potency they are in large part flying blind once they start testing them in humans.
A fairly remarkable example of this is the failed UNITY Biotechnology trial of their lead senolytic for osteoarthritis. From human studies, we know that people suffering with osteoarthritis are burdened with high numbers of senescent cells in their knee cartilage — but obviously, the doctors running the trial weren’t going to take before-and-after biopsies of already worn-down cartilage to see if the drug lowered senescent cell burden. Instead, they tried to pump fluid into the knee via a fine needle and then siphon off and evaluate any change in SASP factors in the fluid that came out the other end. But despite the inside chamber of the knee being a small, tightly-enclosed, presumably senescent-cell-packed space, they still couldn’t get a clear read on whether the SASP was better, worse, or unchanged after therapy. Gathering the same SASP factors out of a blood sample is even less likely to tell you anything about your senescence burden.
A reliable, noninvasive biomarker of senescent cell burden would therefore unlock the potential of the senolytic field. It would give scientists, startups, and investors more confidence in the early stages of human testing that candidate senolytic drugs could actually destroy substantial numbers of senescent cells in living humans, and not just in laboratory mice or human cells in a Petri dish. Being able to validate “target engagement” this way would facilitate clinical trials of senolytics, prevent costly late-stage failures, and de-risk companies for investors, encouraging more funding for companies with viable senolytic candidates. (Unlike generic “biomarkers of aging” such as epigenetic aging clocks, similar biomarkers that reflect the level of specific forms of cellular and molecular aging damage are also important to the development and clinical use of other rejuvenation biotechnologies).
Markers in the Making?
After ruling out the SASP factors themselves as markers of senescent cell burden, there remain a small number of potential candidates for either biomarkers for total senescent cell burden, or for the senolytic action of drugs and other ApoptoSENS therapies such as the engineered senescent cell killers we are working on at SENS Research Foundation. But I’m not confident in the usefulness of any of these candidates today, even if you wanted to gamble on the possibility that they’ll turn out to be reliable measures in humans.
Additionally, you had wanted a test that was readily available with routine blood test screening, and none of the candidates we’ll discuss here are nearly as easy to access as a blood lipid or thyroid hormone test.
A Not-So-suPAR Test
One potential test is the soluble form (suPAR) of urokinase-type plasminogen activator receptor (uPAR), which is released into the serum when it is cleaved off of its normal position on the surface of the cell. When present on the cell surface, uPAR is itself a tricky actor: signaling through the receptor promotes the breakdown of the support structures around the cells in a variety of conditions, including both wound healing and invasion by cancer cells. And when something binds to uPAR to activate it, suPAR is cleaved off and released into the local environment in the process — and some of it reaches the serum.
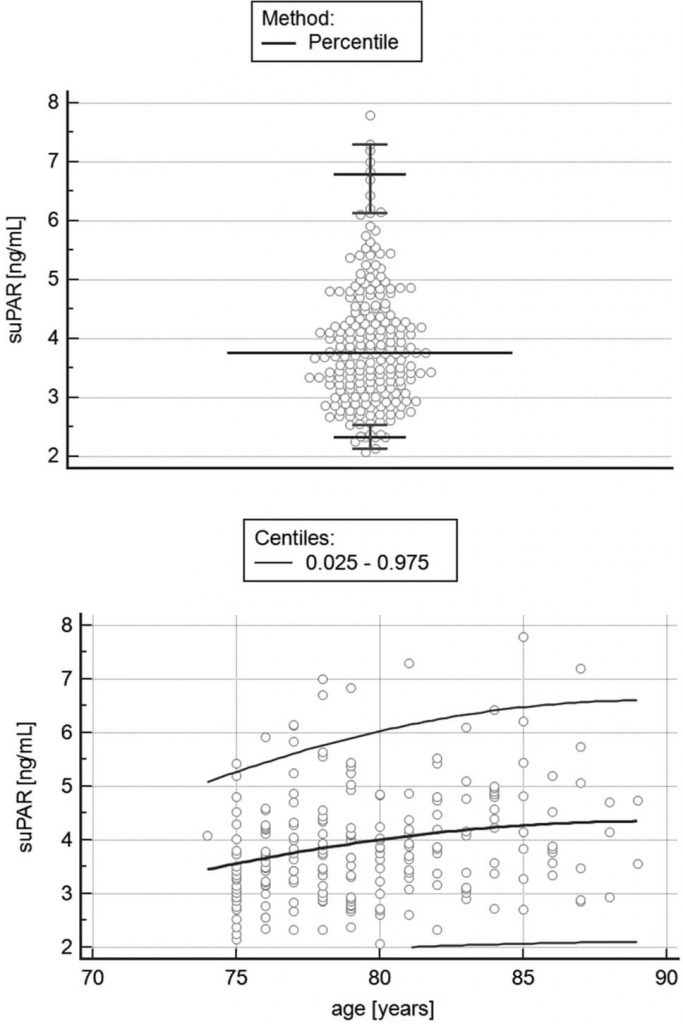
Since suPAR is also a constituent of the SASP, scientists at the Memorial Sloan Kettering Cancer Center wondered if it might function as a senescent cell biomarker. They noted that other researchers had reported that people with a range of diseases in which senescent cells play a prominent role have elevated uPAR and/or suPAR levels, including diabetes, osteoarthritis, and idiopathic pulmonary fibrosis (a terrible disease of the aging lung), and that suPAR is actually used as a severity biomarker in both diabetes and kidney disease. Although it’s only available for research purposes in the United States, there’s even a commercial test for suPAR geared toward using it as a biomarker for the progression of many of these diseases. It is furthermore known that suPAR levels rise with age, although the slope of the rise with age is gentle, the average varies considerably, and it ultimately tends to plateau.
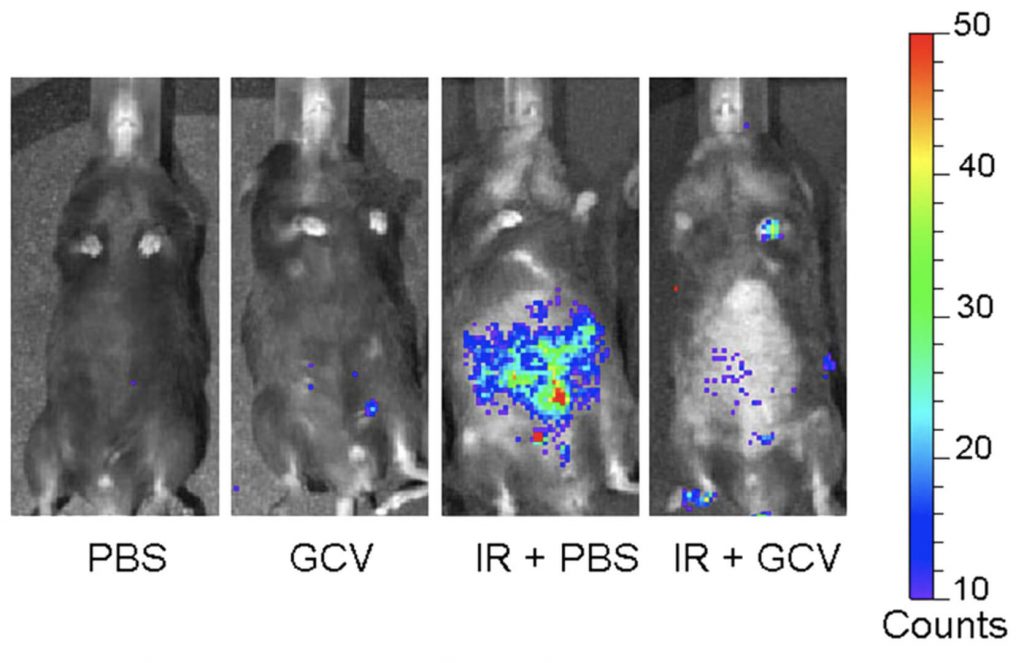
Moving to the laboratory, the researchers found that both the number of uPAR-positive cells and serum suPAR levels increased in mice that were exposed to either chemotherapy drugs that work by forcing cancer cells into senescence, or to a liver toxin that also is known to raise the burden of senescent cells. They also found high levels of uPAR expression in fibrotic human livers that they separately confirmed to be loaded with high numbers of senescent cells, and in atherosclerotic plaques removed from human patients.
Unfortunately, there are a number of reasons to skeptical of the conclusion that suPAR could serve as a good biomarker for senescent cells or SASP. On the mousey side of the question, many of the key experiments in the mouse paper (which, so far, is the only study reporting suPAR as a senescent cell biomarker) used only 3 mice, or in one case 4, for each group, which is just too few animals on which to draw robust conclusions. Additionally, the investigators relied on just one independent senescence marker to confirm the correlation between senescent cell burden and uPAR or suPAR levels. This makes all their claims questionable, precisely because there is no single marker that can identify a cell as senescent. For this reason, it’s expected in the field that all claims about cells being senescent in general and senescent cell burden in particular will be confirmed by testing a sample for multiple senescence markers to confirm that they all line up.
Another problem, which is particularly important to developing longevity therapeutics, is that all the animal studies were actually conducted in young mice. These animals had (or were expected to have) high levels of senescent cells in their tissues not due to the tissue-ravaging effects of the degenerative aging process, but because they’d been subjected to insults that rapidly riddle one’s tissues with senescent cells, including a DNA-damaging chemotherapy drug, cancer-promoting genetic engineering, or being overfed to develop metabolic-associated fatty liver disease (MAFLD). Although the cells were still senescent, the context of that senescence — being in an otherwise-young body with one extreme source of senescence — might well make a suPAR signal clear in these artificial animal models but confounded or swamped out in otherwise-healthy aging humans.
Indeed, while the authors noted that suPAR levels are high in people suffering with a variety of diseases of aging in which senescent cells play a causal role, it’s also elevated in a lot of conditions that don’t seem likely to have such a connection, including many infectious diseases; pancreatitis; mental disorders such as depression, bipolar disease, and schizophrenia; and in young and middle-aged adults (though not people who are biologically old in our current condition) being female rather than male. That last one is notable given that females live longer than males across almost all mammalian species on which we have good information, and especially of human women vs. men. So if suPAR levels are a surrogate marker of senescent cells or SASP burden, why would even relatively young women have higher circulating suPAR?
The weaknesses of the animal evidence; the many diseases unrelated to aging with which elevated serum suPAR levels are associated; the gender paradox; and the fact people of similar ages show a wide range of suPAR levels and the rise with age shows such a gentle and plateauing slope (see the Figure above) compared to the sharp rise in disease and debility, all suggest that suPAR is not likely to be useful as a senescence biomarker.
Paradox in the Plasma
First things first: we’re talking about GDF-15, which is not to be confused with GDF11, the controversial serum factor purported by some to have regenerative effects in aging mice but which has been widely reported to have no such effects and even to cause tissue atrophy instead. GDF-15 is a member of a family of proteins often associated with both inflammation and tissue growth, and is elevated in many pathological conditions (including cancer and the immediate aftermath of a heart attack) and is particularly useful as a marker of inherited mitochondrial diseases.
More to our point, GDF-15 a nonclassical component of the SASP, and at more moderate levels is linked in many, many prospective studies to risk of multiple adverse age-related outcomes, and is a marker of age-related mitochondrial dysfunction as well as inherited mitochondrial disease. All of this seems like circumstantial evidence that elevated GDF-15 might be a biomarker for the SASP. Unfortunately, any such use is confounded by many other things that also elevate GDF-15 and are clearly not related to senescent cells or SASP. The most paradoxical of these is that GDF-15 levels are elevated both by the metabolic syndrome, and by the drug metformin, which of course is used to treat metabolic syndrome and diabetes.
Even more paradoxically, when metformin use leads to weight loss (which it does in some people), it actually seems to work by boosting GDF-15 levels in rodents and in humans. In fact, there’s evidence that the metabolic benefits of weight loss and exercise depend on GDF-15. Metformin and exercise both seem to increase the level of circulating GDF-15 by increasing the production of the stress-response protein ATF4 — which makes the GDDF-15 connection even more paradoxical, because multiple interventions that slow aging in mice also elevate ATF4, and there’s evidence that ATF4 is playing a causal role in the process.
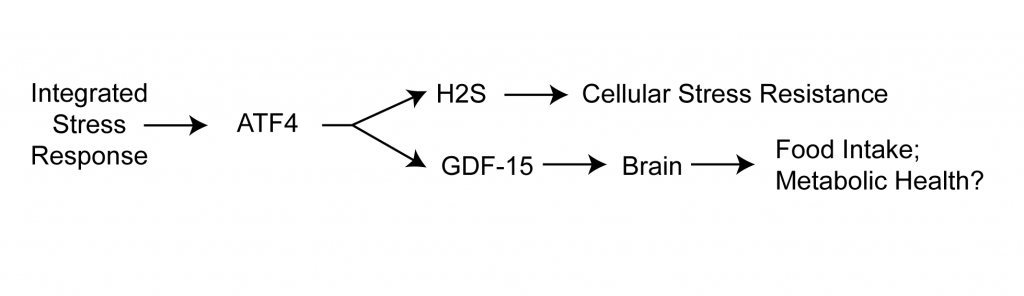
A plausible narrative would be that in all of these cases, GDF-15 is playing a role in responding and adapting to stressors including metformin, exercise, and these various anti-aging interventions; that’s consistent with GDF-15’s role in resolving inflammation. But then where does that leave GDF-15 as a marker of the (inflammatory) SASP? It may well do both (senescence is, after all, itself very stressful state for the cell), but then how do you tell which of these many reasons explains your high GDF-15 level, or even if you should be alarmed or relieved if your levels are high?
Smelling Blood
Although it’s not quite the kind of marker you were looking for and is the furthest from being readily available at a local blood lab, it’s also worth mentioning dihomo-15d-PGJ2 (d15P). This analyte is an eicosanoid (a signaling molecule derived from essential fatty acids) that appears to be uniquely produced inside senescent cells. It’s known that senescent cells undergo metabolic shifts that encourage the production of high levels of eicosanoids, and they do produce some specific fatty signaling molecules as part of the SASP.
But d15P is different: senescent cells produce this unusual lipid mediator, but then hold it inside themselves. It turns out that d15P plays a role in both pushing the cell into the senescent state, and keeping it there — and also in promoting many components of the SASP. This turns into a self-reinforcing cycle, in which d15P enforces senescence through the cell fate regulator p53 — but then p53 promotes the production of a critical enzyme involved in eicosanoid synthesis, which sustains the production of d15P.
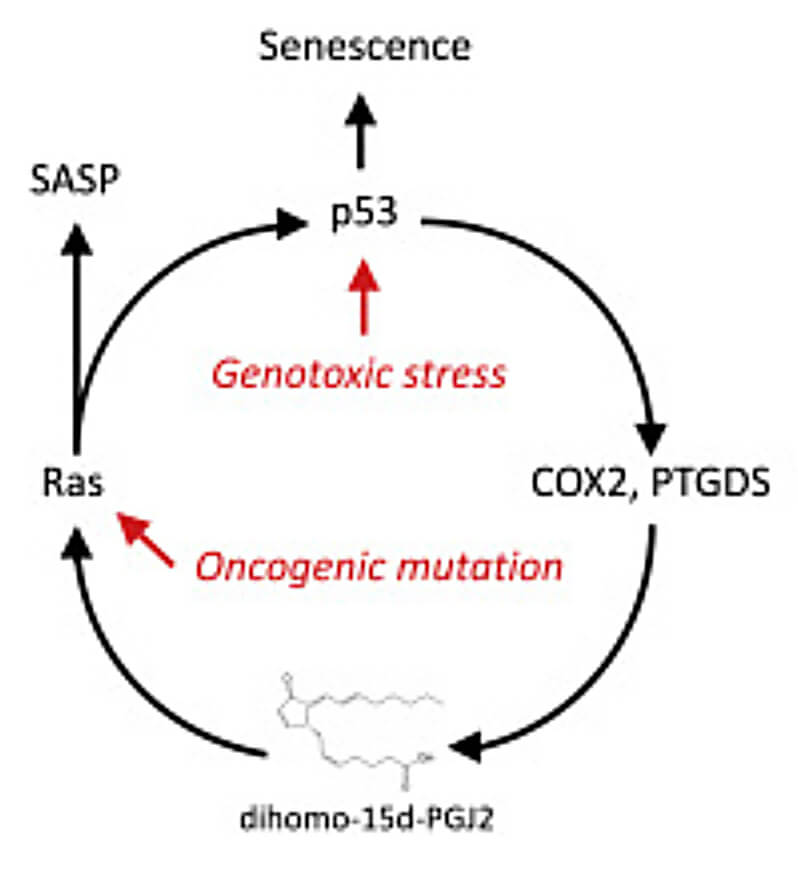
But, you ask, if almost all of a senescent cell’s d15P remains trapped inside of it, how can d15P be used as a blood biomarker for the SASP? The answer is: it can’t. But it can be used as a marker of senolysis: the destruction of senescent cells, through senolytic drugs or engineered senescent cell-killing immune cells.
In the paper reporting these results, the scientists showed that destroying cultured senescent cells with senolytic drugs in a dish causes them to flood the culture medium with d15P, whereas d15P is absent from the medium after destroying non-senescent cells with drugs that induce cellular suicide indiscriminately in all cells. They also confirmed the effect in mice with a high burden of senescent cells. Following administration of senolytic drugs to these mice, d15P levels rose to high levels in their urine and blood in the hours after their senescent cells started to die off. By contrast, no d15P appeared in the fluids of mice with low (young) levels of senescent cells even after the senolytic drug was administered, and or when mice with a high senescent cell burden were given a “placebo” instead of a senolytic drug.
In the near term, a marker that scientists could use to confirm that a potential senolytic drug is actually killing senescent cells in an aging or ill person would be incredibly useful for longevity therapeutics companies working in the senolytic space. Such a marker would enable them to verify that their drugs actually work in people, not just in mice or cells in a dish. That would save a significant amount of potentially wasted money, and also give initial evidence of efficacy – factors with enormous potential to entice major investments from well-financed venture capital firms or Big Pharmas. Then, once these drugs reach the market, the marker would allow future longevity doctors to confirm that their patients were actually benefitting from taking the drugs, and help them decide how often they would need to repeat the therapy.
Unfortunately, like the other potential biomarkers, d15P has some limitations. One is how little work scientists have done to confirm that d15P is a useful senolysis marker under conditions reflecting those used in clinical trials and in medical practice. The mouse studies described above induced senescence by ravaging the bodies of young mice with a senescence-promoting chemotherapy drug, not by testing senolytic drugs in mice with high levels of senescent cells due to aging or disease. A separate study that used d15P to confirm the senolytic effect of a potential senolytic metabolite similarly conducted their test after inducing high levels of senescence in young mice.
Additionally, while many studies show that both suPAR and GDF-15 levels are elevated in people with conditions where senescent cells are implicated, there are no in vivo human data for d15P: nearly everything we know about it comes from these limited cell and mouse studies.
And while it’s difficult to get a person’s suPAR or GDF-15 levels measured, there are at least specialized laboratories that offer the service for human use in scientific studies. By contrast, there is no test even validated to measure d15P in human plasma. Furthermore, in the d15P paper they initially measured d15P using an antibody-based technology that underlies many common laboratory tests. However, the antibody on which this test relies is not specific to d15P, and required the researchers to confirm key results using an additional backup test that requires the more specialized mass spectrometry method. The scientists involved also highlight how understudied d15P is compared to other eicosanoids, and the sheer finickiness of the assay.
So before d15P gets put into human use, a specialized laboratory will first have to develop such a test. And because it would be the first of its kind, there’s not yet a standard against which to validate such a novel test. And since everyone over the age of 30 or so has some non-trivial number of senescent cells, to give meaningful information about how much senolytic impact a given level of d15P represents would require extensive testing in a wide range of people of different ages and with various senescence-linked diseases. Without that additional information, it’s impossible to know whether a given level of d15P following administration of a senolytic means you’ve cleared out an enormous burden of senescent cells, or a few such cells unlucky enough to be vulnerable to a minimally-effective drug.
Navigating by the Stars
As we’ve seen, there’s no reliable marker available for SASP, senescent cells, or successful senolysis — let alone a readily-available lab test that you could expect a health insurer to cover as part of your care. That might leave you feeling that you’re left sailing blind into the sea of your own aging — as, unfortunately, we all by and large are.
But there is some light from the stars by which we can navigate our way home. While we await the availability of biomarkers of senescence, we do know about some important ways to avoid worsening our burden of senescent cells, whatever it may be: not smoking, consuming moderate or no alcohol and other contributors to liver fibrosis, and maintaining a healthily low level of body fat.
And by promoting, funding, and advancing rejuvenation research, we can help steer our ship out of the storm. In the coming safe harbor, our old timbers can be replaced, our hulls scraped clean of barnacles, and our sails mended good as new.



