It was a bit of a mystery to the scientists investigating the phenomenon: a brain disease driven by the death of specialized neurons was strongly linked to exposure to a particular pesticide. Why, then, didn’t exposing those same neurons directly to that same pesticide seem to affect them?
Parkinson’s disease (PD) is a neurodegenerative disease of aging, whose most obvious symptoms — tremors, gait disorders, and a “mask-like” facial appearance — involve the loss of fine motion control. These symptoms are the result of the loss of specialized cells in an area of the brain called the substantia nigra pars compacta (SNc) that specialize in producing the chemical signal-molecule dopamine and are responsible for turning off excess firing of neurons that control muscles. Once a critical number of these “dopaminergic” SNc neurons are lost, the unbalanced firing of those neurons begins to manifest itself in the main motion-related symptoms of the disease.
In all but a few people with rare mutations, degenerative aging processes (such as the accumulation of mitochondrial mutations in SNc neurons) are primarily responsible for the disease. But lifestyle and environmental factors also damage these neurons, and thus increase the risk of a person developing the disease clinically within a currently-normal lifetime. A striking example of this is MPP+, a well-established neurotoxin that specifically attacks the SNc dopaminergic neurons in lab mice, monkeys — and in humans: MPP+’s parent compound, MPTP, has caused numerous cases of Parkinson’s-like syndrome in young people exposed to it in underground drug labs, or via contaminated street drugs.1
So what other exposures might similarly accelerate the degenerative processes that leads to PD? For a long time, scientists have focused on paraquat, a neurotoxic pesticide banned in the EU in 2007, and subject to restricted use in the United States. Paraquat was originally restricted because it can cause lung damage when workers are exposed to high levels of it in the air, but scientists studying it also noted that it has a strong structural resemblance to MPP+. And sure enough, under some conditions it can cause a Parkinson’s-like syndrome in laboratory animals,2 and a strong and consistent relationship has been found between on-farm exposure to paraquat in farm workers (as opposed to exposure to residues in one’s food) and risk of PD.3
Yet, puzzlingly, paraquat doesn’t seem to be particularly toxic to dopaminergic neurons when tested directly; much of the rodent data that seems to show such an effect is ambiguous or unlikely to reflect paraquat exposures actually present in the brain.2 So what might be going on?
As it turned out, the scientists were looking in the wrong place, much like characters in a zombie movie focused intently on the doors when the zombies are about to break through the windows. Paraquat, it turns out, doesn’t directly kill dopaminergic neurons. Instead, it acts by deranging the cells that are supposed to support and nourish them. Meanwhile, the same thing goes wrong in the aging brain, bringing one more insult to the accumulating cellular and molecular damage that slowly erodes the functional capacity of the aging brain, culminating in Parkinson’s and other degenerative syndromes.
The lesson here isn’t just “avoid exposure to dangerous pesticides.” The same study that revealed this surprising indirect mechanism of paraquat’s neurotoxicity also showed how much of the harm can be blocked, and in doing so revealed a new tool in our toolbox for taking the “normal” Parkinson’s disease of aging out of our futures forever.
A Toxin That Makes Zombies Out of Astrocytes
Astrocytes are a kind of support cell for the neurons in the brain. They provide a source of nutrients, maintain the equilibrium in the fluids that surround the neurons, participate in neural repair, and take up and release brain messenger-molecules. Scientists discovered several years ago, however, that rising numbers of astrocytes in the aging brain become senescent. Senescent cells are like cellular zombies: not exactly dead, but not exactly alive any more either, and deadly to all those around them. Senescent cells lose their normal function in the tissue, cease dividing, and begin secreting a deadly mix of inflammatory and tissue-degrading factors collectively known as the senescence-associated secretory phenotype (SASP) that damages and deranges local tissues. Evidence has accumulated to show senescent cells involved in everything from atherosclerosis, to osteoarthritis, to diabetes, cataracts, and on and on.4
It was no surprise, then, when scientists found that the burden of astrocytes with tell-tale signs of senescence rises with age in the brain5 and even faster in those with Alzheimer’s disease.6
This observation got long-time Parkinson’s researcher Dr. Julie Andersen, senescent cell pioneer Dr. Judith Campisi, and their teams at the Buck Institute wondering: could the same be true of Parkinson’s disease? And if so, could it be part of the explanation for the effect of paraquat? And what are the therapeutic implications of such findings in aging people not exposed to this neurotoxin?
Sure enough, when the researchers examined the brains of PD patients, they found more cells exhibiting signs of senescence than in people without the disease — and especially astrocytes, as they had expected.7 This was true even after matching patients for age, meaning that PD subjects had even more senescent astrocytes in their SNcs than is typical for people their age (ranging in this case from 50–92 years at autopsy) — and remember, aging already drives an increase in the burden of these cells as compared with young people, even in those who have yet to develop Parkinson’s disease.7
So, could senescent astrocytes be the link between paraquat and PD? To investigate this possibility, researchers exposed human astrocytes derived from “reprogrammed” human cells to paraquat. In response, the cells underwent changes indicative of senescence, ceasing cell division and churning out key SASP factors.7 Notably, astrocytes were much more vulnerable to paraquat than skin cells: astrocytes went senescent at a lower concentration of the toxin than was required for skin cells, and the doses that turned skin cells senescent killed astrocytes outright.7
So if aging people accumulate senescent astrocytes in their brains, and if an even higher burden of these cells is linked to Parkinson’s, and if even low doses of paraquat turn astrocytes senescent, then might senescent astrocytes be contributing to Parkinson’s disease? To find out, the team looked at the effects of astrocytes on dopamine-producing neurons as mediated by the SASP. When they harvested SASP-laden culture medium from cells rendered senescent by paraquat and transferred it over to separate cultures of neurons, the senescent cell secretions suppressed the ability of mature dopaminergic neurons to survive, and of neural stem-like cells to reproduce and migrate. None of these toxic effects were observed when the same cells were treated with culture medium from non-senescent astrocytes that had not been subjected to paraquat.7
Making “Zombie Cells” Self-Destruct
So far, all of this work on the effects of paraquat on astrocytes — and the havoc those paraquat-exposed astrocytes wreak on neurons — was restricted to cell culture. To see if those effects might explain the link between paraquat, aging, and PD in living organisms, the team turned to genetically altered mice that the Campisi lab had developed for senescent cell studies. Using these mice gives scientists two important abilities: first, to easily track the burden of senescent cells in the mouse’s tissues and how it is affected by aging, environmental exposures, and anti-senescent-cell therapies; and second, to eliminate those cells at will using an inducible “suicide gene.”
Think of this gene construct as a self-destruct device. The gene remains completely dormant and harmless in a mouse’s cell until it is “armed” by the activation of the key senescence gene, p16INK/4a, which rarely occurs except in senescent cells. But once “armed” by p16INK/4a, the device is set to go off as soon as it gets the signal from a remote-controlled trigger. In this case, the trigger is a drug that interacts with the protein produced by the “suicide gene,” which metabolizes it into a form deadly to the cell, causing it to commit programmed cell death (apoptosis).
Consistent with their previous studies in cell culture, mice exposed to paraquat under realistic conditions developed an increased burden of senescent cells in their brains (Figure 1, (a-c)) — and this plague was almost entirely restricted to astrocytes (Figure 1 (d)).
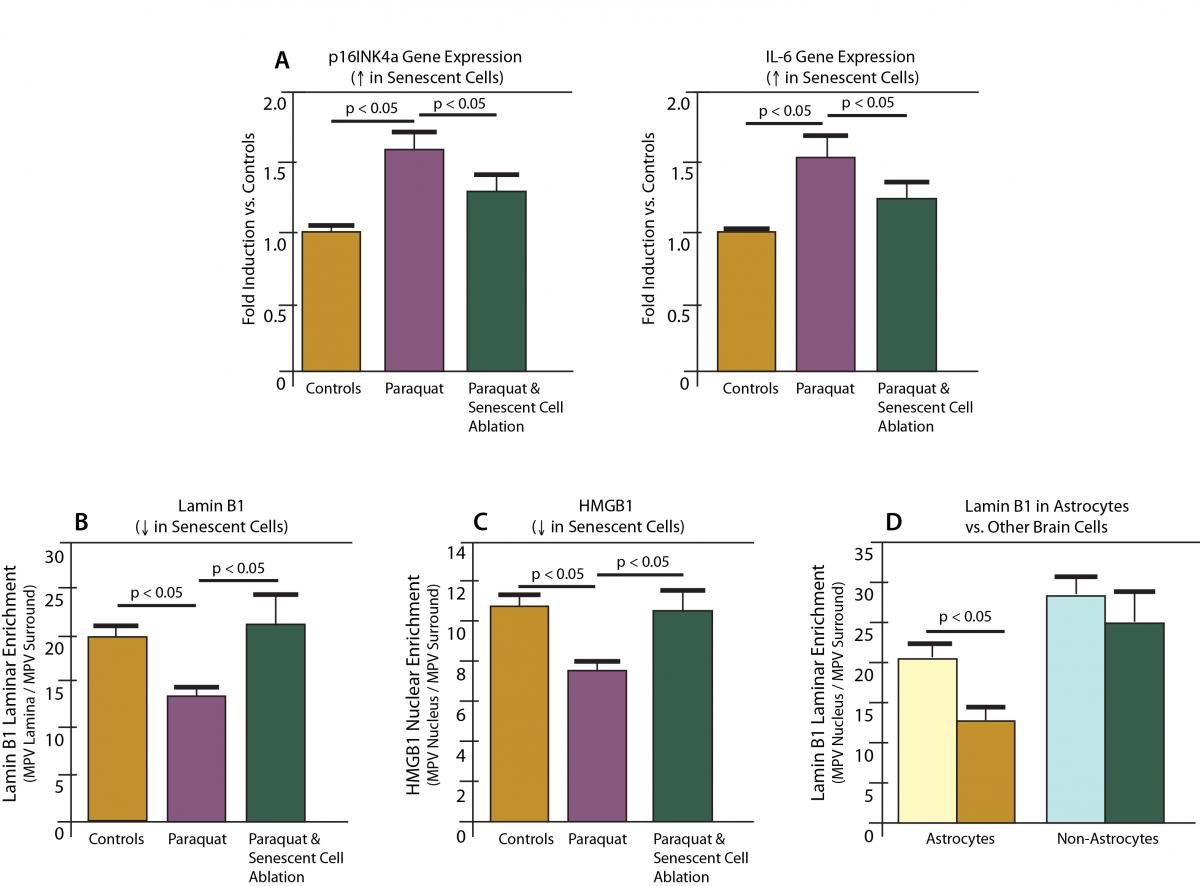
Figure 1. Paraquat induces senescence in astrocytes in mice; ablation by “suicide gene.” Redrawn from (7).
And sure enough, this increased burden of senescent cells caused the same problems in living mice as in the researchers’ cell culture experiments, as well as in previous work in paraquat-exposed mice. These mice developed hallmark signs of PD in their brains and behavior: loss of dopaminergic neurons in the SNc, impaired generation of new neurons in one of the few regions capable of producing them in adult organisms, and impaired muscle coordination similar to human victims of Parkinson’s (as evidenced by difficulty in rearing up on their hind legs) (Figure 2).
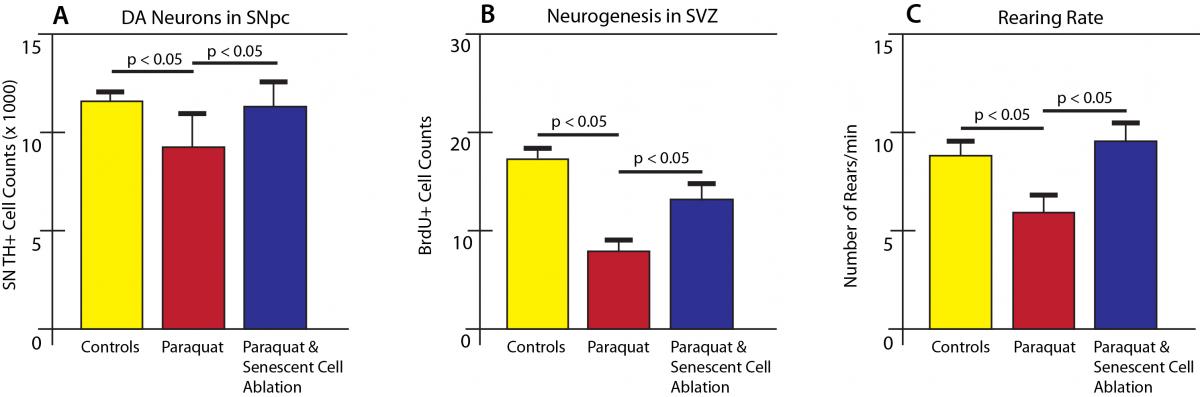
Figure 2. Paraquat-induced senescence is associated with signs of Parkinson’s disease in mice. Rescue by ablation of senescent cells. Redrawn from (7).
But remember, scientists have known for decades that paraquat causes PD-like loss of neurons and symptoms in mice (and humans). How might one prove that the newly-discovered induction of senescence in astrocytes was responsible for the damage, and not some other direct or indirect effect of the toxin? The “damage-repair” heuristic of SENS suggested eliminating the senescent cells themselves, and seeing if that was enough to block the downstream mayhem.
To test this notion, the researchers put paraquat-exposed mice on a regimen of the drug serving as a trigger for the suicide device now armed in all their senescent cells, rapidly eliminating the excess senescent astrocytes from their brains (Figure 1, (a-c)). Sure enough, taking senescent cells out of the picture also eliminated the other harmful effects of paraquat: the loss of dopaminergic neurons and the suppression of new neuron generation was prevented, and the mice were able to rear up normally (Figure 2).
But all of this is about the effects of an agricultural pesticide on the brain, which immediately presses the question: does it really tell us anything about how senescent cells contribute to “regular” PD, driven by the intrinsic aging processes responsible for the great majority of cases of PD? It seems that the answer is likely ‘yes.’ Remember first the circumstantial evidence: the researchers not only found that the burden of senescent astrocytes rises with age in the human brain, but that there is a further excess burden in the brains of people who died with PD. Beyond that, Dr. Andersen has shown in unpublished work (presented at the 2014 Rejuvenation Biotechnology Conference8) that eliminating senescent astrocytes confers similar benefits to mice with a model of PD that more closely mimics the fundamental processes that drive Parkinson’s in aging people not suffering from either environmental toxicity or rare, genetic causes of PD. These mice accumulated more senescent astrocytes in their brains with age, along with dopaminergic neuron death and loss of motor coordination. And just as in the case of the paraquat model, all of these effects were greatly reduced or prevented entirely when senescent astrocytes were ablated by the suicide-gene activating drug.8
The First Zombie-Cell Slayers
Unfortunately, you and I were not born with a “suicide gene” built into our cells that we can activate with a trigger drug, so we need a different solution to eliminate senescent cells. But fortunately, the first such rejuvenation biotechnologies are coming on fast.
In recent years, researchers have developed so-called “senolytic” drugs that wipe out senescent cells in aging mice and mouse models of age-related disease, exploiting the high dependence of these cells on specific biochemical survival pathways.9,10 In these studies, senolytic drugs have restored exercise capacity9 and formation of new blood and immune precursor cells11 in aging mice to near youthful norms, and prevented or treated mouse models of diseases of aging like osteoarthritis,12 fibrotic lung disease,13 hair loss,14 atherosclerosis,15,16 and age-related diseases of the heart itself.9 UNITY Biotechnology is leading a growing charge toward the clinic, with human clinical trials expected to begin in 2019. Based on their work in animal models and with studies in knee tissue from humans with osteoporosis, the company plans to use osteoarthritis as the first of several labeled disease indications (see Question Of The Month #3: Making SENS Part of Medicine), to be followed by cataracts, atherosclerosis, and fibrotic lung disease.
SENS Research Foundation and the Next Generation of Senescent Cell Ablatives
Senolytic drugs are exciting, and could yet prove to be the first rejuvenation biotechnology that becomes widely available to the public. Still, we must be realistic in acknowledging the limits of these therapies and continuing to pursue additional avenues of research to account for said limits.
As noted earlier, senolytic drugs are only able to effectively kill senescent cells while sparing normal cells because senescent cells are much more reliant than healthy cells on the activity or expression of specific genes involved in cell survival. But while this is true of normal cells in most unstressed conditions, normal cells also rely on those same pathways to carry them through times when the cell is under stress, and to give them time to recover afterward. Thus, although the net effect of these drugs is undeniably positive, their mechanism of action will necessarily entail occasionally killing off healthy cells at a moment of vulnerability that they could otherwise have survived, including difficult-to-replace cells like heart muscle cells and (ironically) neurons. Improved rejuvenation biotechnologies would target senescent cells more selectively, and SENS Research Foundation is helping to advance those next-generation “senoablatives” even as UNITY prepares for human testing.
One such rejuvenation biotechnology is under development by Oisín Biotechnologies, a startup launched in 2015 with seed funding and access to intellectual property from the Foundation and our allies at the Methuselah Foundation. Oisín’s technology is actually a variation on the “suicide gene” strategy used in the senescent astrocyte study7 and similar systems used in previous proofs-of-concept, including the first breakthrough in the field. But in this case, you don’t have to be born with the “suicide gene” already in your cells to benefit from it. Instead, the “suicide gene” is delivered to aging tissues using a kind of gene therapy.
Unlike the case of most gene therapies that are intended to permanently alter their target cells, however, Oisín’s senoablative genetic constructs will not be inserted permanently into the patient’s genome: instead, its genetic payload will be expressed temporarily from the main body of the cell, following which the construct will be passively degraded by normal cellular metabolism.
After initial testing demonstrated that their senoablative constructs could eliminate up to 80% of senescent cells in cell culture, the Oisín team tested them out in living mice, showing that it can purge senescent cells from multiple tissues in middle-aged animals.
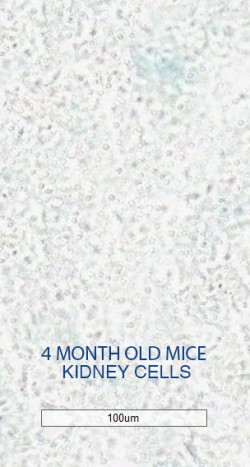
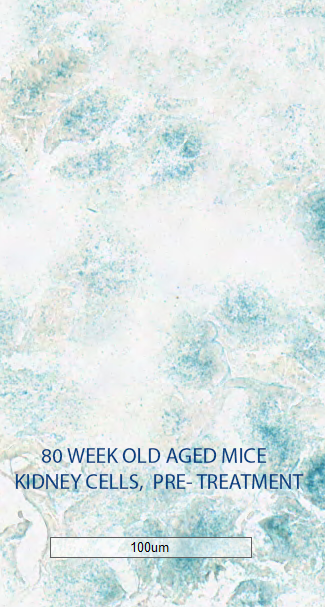
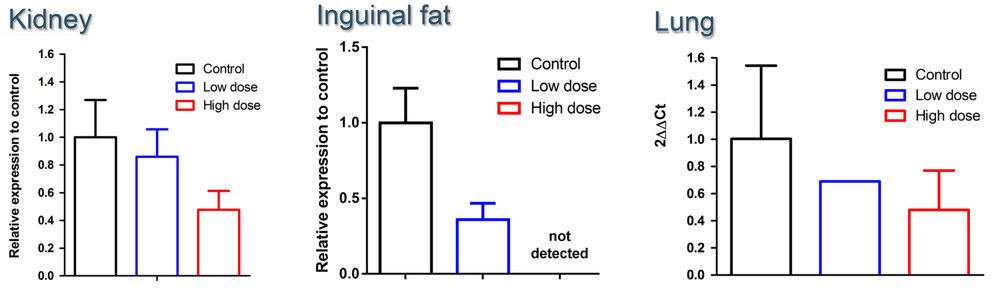
Figure 3. Oisín senolytic gene construct clears senescent cells from aging mouse tissues. From the Oisín Biotechnologies website.
In addition to being much more narrowly-targeted to senescent cells than the senolytic drugs, this system also has several advantages over gene therapies that would be permanently inserted into your cells. The most important such advantages relate to safety. The tools currently available to insert new genes permanently into cells do so at a random site inside the cell, which occasionally results in the disruption of the cell’s native genes at the site of insertion, which could potentially turn the cell cancerous or cause other problems. This is why SENS Research Foundation is working on new ways to safely deliver large genetic payloads to our cells. In the meantime, Oisín’s transient gene expression technology essentially eliminates this risk by not requiring insertion into the cell’s own genome.
Additionally, the fact that Oisín’s “suicide gene” is only present in tissues temporarily means that if any side effects were to emerge, the treatment is rapidly self-extinguishing. Its time-limited activity also allows therapy to be be withdrawn at times when a temporary rise of senescent cells in a tissue is needed as part of physiological processes (such as in resolving fibrosis, in wound healing, and even in pregnancy). Afterward, senoablative therapy can be initiated again to mop up any senescent cells not eliminated by physiological processes, and to resume clearance of the backlog of senescent cells that existed before therapy was temporarily suspended.
Breaking Down the Barricades
In addition to drugs and gene constructs, there’s another strategy in the works for eliminating senescent cells: enhancing the innate immune system’s ability to purge them. Studies over the course of the last decade have revealed the somewhat surprising fact that the body’s immune system routinely eliminates senescent cells from our tissues. While several components of the immune system are involved, the key players are natural killer cells (NK cells). But then why do senescent cells accumulate in our tissues with age? Do aging processes lead to a decline in an initially-robust senescent-cell clearing capacity? Or do NK cells never quite catch all the senescent cells that arise at any given time, and the few that remain slowly accumulate over time? Or are a few senescent cells particularly resistant to immunological clearance? And most importantly: can this innate immune clearance of senescent cells be made more effective, so that our bodies can purge themselves of these zombie cells, without requiring an exogenous treatment?
We announced last year that with the support of the Forever Healthy Foundation, we would be funding a new project in Dr. Campisi’s lab to explore these questions and look for solutions. We are also now preparing to expand this project with a complementary intramural project at our Research Center in Mountain View, California.
Each lab will focus on its strong suit. With their deep expertise in the biology of senescent cells, the Campisi lab will be focused on fundamental research into questions like how senescent cells vary in their susceptibility and resistance to immune clearance (depending on factors like their tissues of residence or the pathway that led them into senescence); the targets and mechanisms used by NK cells to clear senescent cells; and why subsets of senescent cells might persist when their similarly-situated neighbors are cleared out (and what might allow us to overcome that resistance).
Meanwhile, our team at the Research Center will be picking up and running with an emerging mechanism of such resistance, testing one of two different platforms to see if we can deny senescent cells the ability to shield themselves from NK clearance. The details on these complementary projects will be announced later this year.
Progress likewise proceeds apace in rejuvenation biotechnologies targeting the other key aging damage driving Parkinson’s disease specifically. There are now multiple immunotherapies targeting clearance of alpha-synuclein from the brain in early-stage clinical trials, and multiple trials underway or in the works on the next generation of cell replacement therapies for dopaminergic neurons, including the TRANSEURO trial; the Summit4StemCell initiative, put together by Jeanne Loring — a researcher at The Scripps Research Institute who is exceptionally engaged with turning her research into therapies; a Japanese trial to be run by Jun Takahashi of Kyoto University in Japan (cf. here and here); and a trial centered at Memorial Sloan Kettering Cancer Center headed by cell biologist Lorenz Studer.
The benefits of senescent cell clearance to the health and longevity of aging mice have turned out to be more dramatic and sweeping than anyone ever expected. SENS Research Foundation is working to bring those same benefits to aging humans, advancing us to a future where living long is permanently decoupled from age-related debility and disease.
References
- Langston JW. The MPTP Story. J Parkinsons Dis. 2017;7(s1):S11-S22. doi: 10.3233/JPD-179006. PubMed PMID: 28282815; PubMed Central PMCID: PMC5345642.
- Tieu K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb Perspect Med. 2011 Sep;1(1):a009316. doi: 10.1101/cshperspect.a009316. Review. PubMed PMID: 22229125; PubMed Central PMCID: PMC3234449.
- Pezzoli G, Cereda E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology. 2013 May 28;80(22):2035-41. doi:10.1212/WNL.0b013e318294b3c8. PubMed PMID: 23713084.
- Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017 Jul;21:21-28. doi: 10.1016/j.ebiom.2017.04.013. Epub 2017 Apr 12. Review. PubMed PMID: 28416161; PubMed Central PMCID: PMC5514381.
- Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, Lu T, Yankner BA, Campisi J, Elledge SJ. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015 Sep 25;349(6255):aaa5612. doi: 10.1126/science.aaa5612. PubMed PMID: 26404840; PubMed Central PMCID: PMC4942138.
- Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One. 2012;7(9):e45069. doi: 10.1371/journal.pone.0045069. Epub 2012 Sep 12. PubMed PMID: 22984612; PubMed Central PMCID: PMC3440417.
- Chinta SJ, Woods G, Demaria M, Rane A, Zou Y, McQuade A, Rajagopalan S, Limbad C, Madden DT, Campisi J, Andersen JK. Cellular Senescence Is Induced by the Environmental Neurotoxin Paraquat and Contributes to Neuropathology Linked to Parkinson’s Disease. Cell Rep. 2018 Jan 23;22(4):930-940. doi: 10.1016/j.celrep.2017.12.092. Epub 2018 Jan 28. PubMed PMID: 29386135; PubMed Central PMCID: PMC5806534.
- Andersen JK. Senescence and the aging brain. Presentation at Rejuvenation Biotechnology 2014 (RB2014). August 21-23, 2014 Santa Clara, California. Parkinson’s Disease Session. Program p. 41. Video Presentation.
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015 Aug;14(4):644-58. doi: 10.1111/acel.12344. Epub 2015 Apr 22. PubMed PMID: 25754370; PubMed Central PMCID: PMC4531078.
- Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IJcken WF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JHJ, Campisi J, de Keizer PLJ. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017 Mar 23;169(1):132-147.e16. doi: 10.1016/j.cell.2017.02.031. PubMed PMID: 28340339; PubMed Central PMCID: PMC5556182.
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016 Jan;22(1):78-83. doi: 10.1038/nm.4010. Epub 2015 Dec 14. PubMed PMID: 26657143; PubMed Central PMCID: PMC4762215.
- Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017 Jun;23(6):775-781. doi: 10.1038/nm.4324. Epub 2017 Apr 24. PubMed PMID: 28436958; PubMed Central PMCID: PMC5785239.
- Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017 Feb 23;8:14532. doi: 10.1038/ncomms14532. PubMed PMID: 28230051; PubMed Central PMCID: PMC5331226.
- Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, Ben-Porath I, Krizhanovsky V. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016 Apr 6;7:11190. doi: 10.1038/ncomms11190. PubMed PMID: 27048913; PubMed Central PMCID: PMC4823827.
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016 Oct;15(5):973-7. doi: 10.1111/acel.12458. Epub 2016 Aug 5. PubMed PMID: 26864908; PubMed Central PMCID: PMC5013022.
- Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016 Oct 28;354(6311):472-477. Epub 2016 Oct 27. PubMed PMID: 27789842; PubMed Central PMCID: PMC5112585.



