Parkinson’s disease (PD) is diagnosed on the basis of what are called “motor symptoms” of the disease, including tremors, rigidity, bradykinesia (slowness of movement), and a “mask-like,” emotionless facial expression. This group of symptoms is caused by the progressive loss of a group of neurons in an area of the brain called the substantia nigra pars compacta (SNc). These neurons produce the chemical messenger dopamine and transmit it elsewhere in the brain to help fine-tune the signaling that allows you to consciously initiate and control movement. So as these neurons lost, such fine control over movement is lost, and the motor symptoms erupt. This is also why levodopa — the chemical building block of dopamine — is the go-to drug for PD: giving the surviving dopamine-producing neurons more building blocks allows them to eke out more dopamine, going a long way toward correcting the subset of PD symptoms that are caused by loss of dopamine-producing cells (particularly in the early stages of the disease).
Unfortunately, levodopa does nothing to slow down the ongoing loss of critical dopamine-producing neurons in the brains of people with PD, or in aging people who have not yet lost enough of these cells to meet the diagnostic criteria for PD. But there’s another group of PD symptoms, termed the “non-motor symptoms” (NMS) of PD, that gets far less attention, even though NMS begin to manifest earlier in the disease, are harder to treat with current therapies, and include some of the most crippling features of living with the later stages of PD.(1,2).
NMS actually usually appear earlier in PD than the motor symptoms do, but at that point they’re so mild — and also so common in aging people — that they usually aren’t recognized as the first signs of the pathology to come, but are instead put down to so-called “normal aging”. Such early-arising NMS include loss of the sense of smell; constipation and other gastrointestinal symptoms; incontinence; and erectile dysfunction. Anxiety or depression can also be an unrecognized part of the early stages of PD. A more PD-specific early NMS is the loss of the body’s ability to turn off its movements while dreaming, resulting in complex movements and speech in the victim’s sleep. But as the disease progresses, NMS become more and more severe, and can ultimately include dementia, hallucinations, and obsessive, stereotyped, repetitive behaviors called punding (Figure 1).
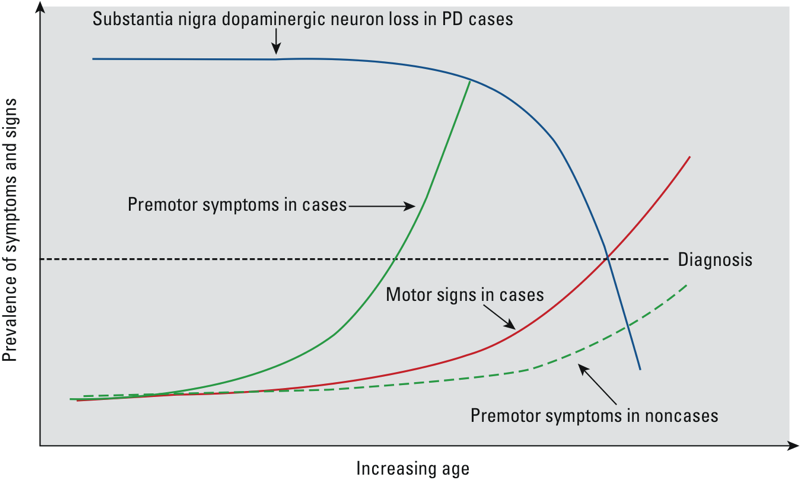
Figure 1. Trajectory of Motor and Early Nonmotor (Here “Premotor”) Symptoms in Parkinson’s Disease. From (7).
Whereas PD motor symptoms are driven by loss of dopamine-producing neurons, many of the nonmotor symptoms are instead linked to the accumulation of Lewy bodies and other malformed clumps and fibrils of the protein alpha-synuclein (AS) inside and between neurons.(1,2) The various forms of aggregated AS first appear in neurons in the periphery of the body (that is, outside of the brain and spinal cord), and then invade the brain, starting from the base of the skull and slowly spreading their way forward across the brain over the course of the disease. Because most NMS are not driven primarily by loss of dopamine signaling, levodopa and related dopamine-boosting drugs are completely ineffective in controlling most of them; in fact, using the drug can sometimes make some NMS worse. Patients can find themselves on a whole pharmacopoeia of drugs that address their NMS on a one-off basis, targeting individual symptoms — sildenafil (Viagra®) for erectile dysfunction, stimulants for daytime sleepiness and fatigue, clozapine for psychosis, and even glycopyrrolate for frank, uncontrolled drooling — but again, these drugs do nothing to check the underlying progression of AS pathology, and the NMS continue to worsen over time.
But as we’ve chronicled in two previous blog posts, there’s now hope: a race amongst several biotech companies to develop rejuvenation biotechnologies to clear AS aggregates out of the aging and early PD brain. These companies are taking what, in SENS terminology, is the amyloSENS approach, developing and testing antibodies that recognize and bind to malformed AS, allowing them to interdict the toxic proteins as they spread from one neuron to the next, and possibly also capturing some of the aggregates inside existing neurons and facilitating their degradation. By sweeping away AS aggregates before they get a chance to spread, AS immunotherapies have the potential to hold the nonmotor symptoms of PD at bay, slowing the overall progress of the disease, and — when combined with mature cell therapy — eventually preventing the disease altogether, and potentially even reversing it.
PRX002: Targeting C-Truncated Alpha-Synuclein
When we last reported news from Prothena Corp PLC, their cofounder and CEO Dr. Dale Schenk had recently presented the results of studies using their AS-targeting antibodies at SENS Research Foundation’s Rejuvenation Biotechnology 2014 conference. Scientists at Prothena had confirmed that several of their candidate antibodies were able to clear AS pathology out of the brains and spinal cords of mouse models of PD and related disorders, substantially shielding them against the PD-like motor and cognitive impairments suffered by their untreated cousins. Schenk also revealed some very early information specific to PRX002, the humanized version of the most promising antibody tested in the mouse studies, which was then slated to enter into early-stage human trials.*
Now we can report on the first published results of those trials,(3) and on early information coming out of an additional trial that has not yet been formally published.(4-6)
In their first-in-human, double-blind, placebo-controlled Phase I trial, Prothena scientists recruited 40 healthy people without PD to receive either placebo injections or or one of 5 doses of PRX002, ranging from 1 to 30 mg/kg. An hour after dosing, the lowest dose of the antibody led to a reduction of AS in the circulation of more than 30%, with the highest doses reducing it by up to 96%; 24 hours later, levels remained similarly suppressed in the higher-dose groups:(3)
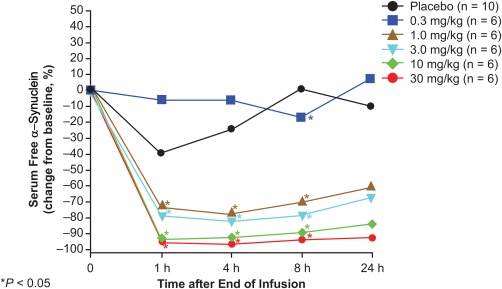
Figure 2. A single dose of PRX002 substantially reduces free alpha-synuclein in serum. From (3).
At the same time, the total AS in serum — including both the remaining free AS and the AS that had been swept up by the antibody — actually increased from baseline, to levels ranging from double (at the lowest dose) to triple (at the highest) what it had been when subjects entered the trial.(3) What, exactly, this means, we don’t know yet. It might be a sign of the “peripheral sink” mechanism, through which the capture of AS in the circulation creates a kind of “osmotic pressure” that draws the more concentrated AS on the other side of the “membrane” of the blood-brain barrier (Figure 3, middle). This would reduce the concentration of AS in the brain, but the circulating level might temporarily increase.
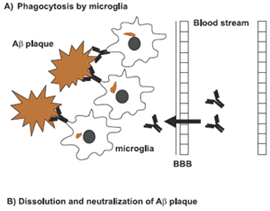
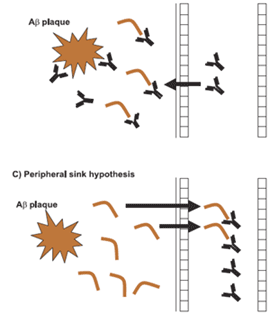
Figure 3. Possible Mechanisms of Aggregate Removal from the Brain. Example is for Beta-Amyloid. From (8).
However, that isn’t how Prothena scientists think PRX002 works: they believe, based on cell culture and in vivo studies, that PRX002 captures aggregated AS directly in the brain, preventing the uptake by neurons. The asyn bound to the antibody is then recognized and taken up by the brain’s immune cells, which degrade the captured AS just as they would a captured pathogen (Figure 3, bottom) (personal communication, Dr. Wagner Zago). Instead, Prothena researchers think the rise in total plasma AS is just a natural consequence of the fact that PRX002 has a much longer half-life in the circulation than does the free form of AS in the circulation, so once an AS molecule is captured in the periphery, it takes a lot longer for it to finally leave the circulation than it would if it were left to itself (personal communication, Dr. Wagner Zago).
The therapy did appear to be reasonably safe: nearly all of the relatively small number of adverse events that occurred during the course of the trial were mild; the six moderate-severity adverse events and the one severe event (a case of neutropenia) were judged to be unrelated to the treatment.(3)
Again, this was only a basic safety and pharmacokinetics trial, and was conducted in healthy young people without PD. We learned more about the potential of PRX002 from a sneak peak at the interim results from an ongoing Phase 1b clinical trial of of PRX002 in PD patients (4) that were presented at the 13th International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD 2017) in April,(5) discussed by Prothena management and scientists in a concurrent conference call with investors,(6) and again presented at the 2017 International Congress of Parkinson’s Disease and Movement Disorders in June.(5a)
This was a larger (80 subjects) trial that for the first time involved volunteers suffering PD — most with the early stages of the disease (Hoehn and Yahr stage I-II) and a few with what is usually called “moderate” PD (stage III). Patients were required to have been stable on PD medication for at least 3 months before study entry.(4-6) Volunteers were randomly allocated to receive three rounds of the antibody (or placebo) at one of six doses over the course of 12 weeks, and then followed for an additional 24 weeks to see how they would fare. This time, Prothena scientists would also look to see if the antibody entered into the cerebrospinal fluid (CSF) bathing the brain and spinal column, although they are not yet able to detect its engagement with AS in the CSF.(4-6)
The participants haven’t completed their followup periods yet, but here’s what Prothena scientists have learned thus far:
First, the effects of the first dose of PRX002 on serum AS in PD patients were similar to what was seen in young, healthy people in the first trial: up to a 97% decline in the ratio of free to total AS at the highest dose, with the ratio remaining strongly suppressed for at least four hours (Figure 4, left). But now for the first time, they could see the longer-term effects of each dose. A month after receiving their first dose, just before taking their second shot, subjects’ free-to-bound AS ratios had only partially returned to where they had been before receiving their first dose. Most notable were subjects who received doses of at least 3 mg of antibody (green line with squares): free-to-bound AS ratios remained suppressed from their first visit in a dose-dependent fashion, to a degree proportional to the initial reduction (Figure 4, left). The effect was even more pronounced by the time subjects returned to the clinic for their third dose of PRX002 on day 57. Having had their second dose a month earlier, and not yet having received their third round of therapy, the volunteers’ free-to-bound AS ratios were as much as 90% lower than they had been at baseline in the highest-dose group (Figure 4, right) (4-6)!
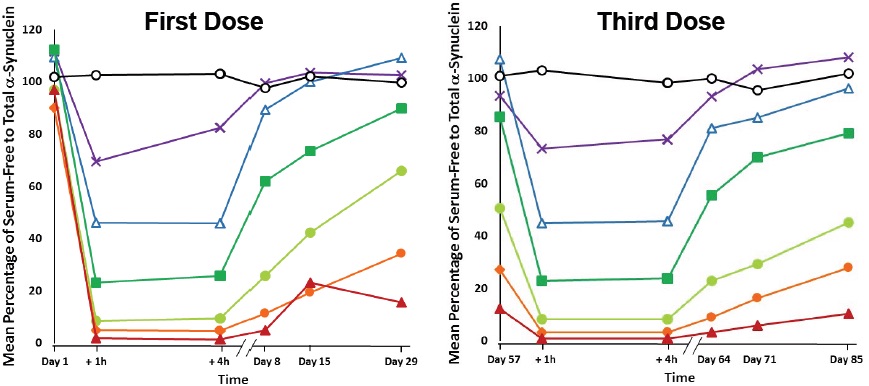
Figure 4. Multiple Doses of PRX002 Progressively Lower Serum Alpha-Synuclein in Serum.(6)
The real action, of course, is in the brain, not the serum, and an additional aim of this trial was to confirm that PRX002 would penetrate the blood-brain barrier. Indeed it did, with CSF concentrations reaching about 0.3% of plasma concentrations at any given dose of the antibody. However, no reduction was observed in the level of unaggregated, “monomeric” AS in the CSF — that is, of individual molecules of AS, as opposed to the assemblies that researchers believe to be toxic to neurons. These AS monomers dominate in the circulation, where they are subject to capture by circulating PRX002. In the brain, however, the situation is reversed, with AS aggregates outnumbering monomers outside of the neurons themselves. The investigators and the company point out that PRX002’s most important feature is its ability to capture and eliminate this more dangerous aggregated AS.
Unfortunately, as noted above, there’s currently no way to measure in AS aggregates in the CSF.† But in Petri dish studies and in experimental animals, PRX002 has over 400 times greater affinity for aggregated than monomeric AS.(6) So it’s entirely possible that the available PRX002 present in the CSF is entirely occupied with binding brain AS aggregates, leaving few molecules of the antibody free to capture the residual monomeric AS.(6) Based on what is known about the behavior of the antibody and the available pharmacokinetic data, Prothena CEO Gene Kinney has stated (albeit without presenting modeling to support the assertion) that “we would expect to fully saturate that pathological load [of aggregated AS] in patients with Parkinson’s disease.”(6)
No serious adverse reactions to PRX002 were observed in trial participants: some people reported skin reactions such as rash at the infusion site, and there were a few reports of constipation, headaches, or peripheral edema. There was no improvement on the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) or other signs of disease progression, but no firm conclusion can be drawn from this due to the short duration of the trial period, especially with so few people getting the higher doses. (Outside Parkison’s experts agreed). Possible effects on other biomarkers and functional scales — including smartphone-based motion sensor data, the level of tau protein in the CSF (which they can measure), and the markers of dopamine metabolism in the brain (although you wouldn’t necessarily expect an effect on that) — are still under analysis.
Based on these results, Prothena is now recruiting 300 subjects with early-stage PD for a year-long Phase 2 trial of PRX002 (called RO7046015 and RG7935 by Prothena’s partner Roche).(6)
BIIB054: Racing Under the Radar
Biotech pioneer Biogen has been rather quiet about their work on their AS-targeting antibody BIIB054 — unlike their widely-heralded Aducanumab, another amyloSENS-style immunotherapy, which has generated enormous excitement for what seems to be the clearest-cut effect on both beta-amyloid and problems with cognitive function in people with Alzheimer’s disease. They initiated their Phase 1 BIIB054 trial in 2015, and reported promising early results of its use in an animal model of Parkinson’s back in the summer of 2016, but have never published the results, issued press releases, or held conference calls to share their findings with the wider public. But all the while, BIIB054 has been jumping one hurdle after another, and the company is powering ahead.
BIIB054 was originally developed by biotech company Neurimmune, using a platform they call Reverse Translational Medicine (RTM®), which derives therapeutic antibody candidates from libraries of de-identified B-cells isolated from ostensibly healthy older adults (those free of specific neurological diseases of aging). The RTM hypothesis is that the immune systems of people who reach the ages at which AS, beta-amyloid, and other pathological aggregates begin to accumulate would generate antibodies against their abnormal form, and that people who remain free of these specific diseases might be producing antibodies that are particularly effective at keeping these aggregates at bay. Antibodies produced by such people’s B-cells were screened first for their ability to bind AS aggregates in vitro, and then for their ability to interdict the cell-to-cell transmission of AS between isolated neurons — a key point at which to arrest the spread of AS pathology acrosss the brain.(9)
BIIB054 emerged from this and subsequent testing as a strong candidate anti-AS imunotherapy. In further testing, BIIB054 selectively bound aggregated AS in tissue samples from people with PD and with Dementia with Lewy Bodies (DLB — a related disease of AS-driven neurological aging), while leaving native AS alone.(10) The company’s scientists also report that when they then injected preformed AS fibrils into the brains of mice, BIIB054 slowed the self-templating spread of AS pathology across the brain (consistent with their cell-culture study), and held much of the ensuing motor dysfunction at bay(9,10) (reducing it “by more than 50 percent” according to a science journalist who was present when they presented results at AD/PD 2017 (11)).
We should be cautious about all of this until we get the kind of details and expert feedback that comes with publication in a peer-reviewed journal article. But Biogen clearly takes their results seriously, and so apparently does the FDA: based on these results, Biogen advanced BIIB054 into a Phase 1 clinical trial in 2015.
48 healthy people without PD, aged 40-65, were given a single dose of BIIB054 at one of six doses across a very wide range, and followed up for the next 16 weeks using multiple clinical and laboratory assessments, as well as MRI and electrocardiogram data.(11) The results reported so far are similar to the PRX002 results as far as they go, but are clearly at an earlier stage. Serum levels of BIIB054 antibody rise steadily with the injected dose,(11) with a half-life of 28 days according to Madolyn Bowman Rogers, a science journalist who attended AD/PD 2017, citing Biogen scientist Miroslaw Brys. Levels in the CSF follow suit, reaching 0.2% of the serum concentration.(11) The same journalist says that Brys indicated that Biogen had not yet completed its analysis of BIIB054’s effects on plasma AS at the time of the conference, but that they had already decided to advance BIIB054 into Phase 2 trials.
Gathering the Champions
This is an exciting moment. Three different amyloSENS rejuvenation biotechnologies have now emerged from the rejuvenation biotechnology ecosystem, targeting the removal of aggregated alpha-synuclein from the brain and being tested in early-to-mid-stage human clinical trials. Each uses a different approach to targeting these malformed proteins, and is supported by data in animal models — and the early human evidence looks favorable, if very preliminary. And there are more in the pipeline, including candidate AS immunotherapies from Proclara, NeuroPore, and BioArctic Neuroscience.
Meanwhile, work is advancing to remove and repair the rest of the cellular and molecular damage driving Parkinson’s as a clinical syndrome of the aging brain. Several clinical trials are either about to begin or are already underway to replace and reinforce the dopamine-producing neurons in the aging brain, building on the promise of the first faltering steps in cell therapy for PD. These include the TRANSEURO trial; the Summit4StemCell initiative; a Japanese trial to be run by Jun Takahashi of Kyoto University in Japan (cf. here and here); a trial centered at Memorial Sloan Kettering Cancer Center headed by cell biologist Lorenz Studer; and another being organized by cell therapy pioneer Ivar Mendez at the University of Saskatchewan and Ole Isacson of Harvard.
At an earlier stage, Julie Andersen, PhD and coworkers at the Buck Institute have reported that destroying senescent inflammatory cells in the brains of animals substantially protects them against paraquat, a toxin that causes a PD-like syndrome in mice and humans alike by selectively killing dopamine-producing neurons in the brain.(12,13) The potential for turning this animal research into a working rejuvenation biotechnology is drawing close. Dr. Andersen’s collaborator Judith Campisi, PhD has established UNITY Biotechnology, a biotech startup focused on drugs that selectively destroy senescent cells. They expect to move their lead candidate UBX0101 into human clinical trials for osteoarthritis sometime in 2018, and move on to other clinical indications after that. Oisín Biotechnology — a startup with initial funding from SENS Research Foundation, the Methuselah Foundation, and others — is close behind them, having developed a form of gene therapy that — in animals at least — also destroys these cells, using an approach that is less inherently likely to destroy healthy cells along with senescent ones. And while it’s early-stage work in an area that still recieves little investment from government and industry sources, Matthew O’Connor’s mitochondrial mutations team at SENS Research Foundation continue their work to create “backup copies” for the genes that encode the proteins of our energy-producing mitochondria.(14) The dopamine-producing cells of the SNc are particularly vulnerable to mutations in their mitochondrial genes, and a role for these mutations in PD is widely recognized.
All this suggests that multiple developments are converging toward groundbreaking progress in this area. One of these therapies will be shown to be safe and effective enough for FDA approval. People in the early stages of Parkinson’s will benefit from these therapies first, having the course of their disease slowed and the quality of their lives extended. Next, people at high risk of the disease will begin to get access, allowing some to postpone the disease all together. And then one after another, rejuvenation biotechnologies that replace missing dopamine-producing cells, destroy senescent cells in the brain, and keep our mitochondrial energy centers humming will become available. Our hands may tremble, reaching for this new medicine — but then no one’s hands will tremble with Parkinson’s disease again.
*Sadly, Dr. Schenk — a pioneering scientist who won the American Academy of Neurology’s 2001 Potamkin Prize for the development of the first immunotherapy targeting beta-amyloid — did not live to see the publication of these results: he died last fall of pancreatic cancer.
†This is obviously a critical tool for future research: the Michael J. Fox Foundation has recently stepped up with a $2 million prize for the first researcher to create a PET tracer for visualizing AS aggregates in the brain).
References
1: Lee HM, Koh SB. Many Faces of Parkinson’s Disease: Non-Motor Symptoms of Parkinson’s Disease. J Mov Disord. 2015 May;8(2):92-7. doi: 10.14802/jmd.15003. Epub 2015 May 31. Review. PubMed PMID: 26090081; PubMed Central PMCID: PMC4460545.
2: Stern MB, Lang A, Poewe W. Toward a redefinition of Parkinson’s disease. Mov Disord. 2012 Jan;27(1):54-60. doi: 10.1002/mds.24051. PubMed PMID: 22252891.
3: Schenk DB, Koller M, Ness DK, Griffith SG, Grundman M, Zago W, Soto J, Atiee G, Ostrowitzki S, Kinney GG. First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers. Mov Disord. 2017 Feb;32(2):211-218. doi: 10.1002/mds.26878. Epub 2016 Nov 25. PubMed PMID: 27886407; PubMed Central PMCID: PMC5324684.
4: Soto J, Prothena Biosciences Ltd, Hoffmann-La Roche. Multiple Ascending Dose Study of PRX002 in Patients With Parkinson’s Disease. Identification No. NCT02157714. Retrieved from http://clinicaltrials.gov/ct2, 2017-05-24.
5: Jankovic J, Goodman I, Safirstein B, Schenk D, Kinney GG, Koller M, Ness DK, Griffith SG, Grundman M, Soto J, Ostrowitzki S, Boess FG, Martin-Facklam M, Quinn JF, Isaacson SH, Jennings D, Omidvar O, Ellenbogen A. Results from a Phase 1b multiple ascending-dose study of PRX002, an anti-alpha-synuclein monoclonal antibody, in patients with Parkinson’s disease. 13th International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD 2017), Vienna, Austria, 02-Apr-2017 11:45 13:45.
5a: Jankovic J, Goodman I, Safirstein B, Schenk D, Kinney G, Koller M, Ness DK, Griffith S, Grundman M, Soto J, Ostrowitzki S, Boess F, Martin-Facklam M, Quinn J, Isaacson S, Jennings D, Omidvar O, Ellenbogen A. Results From a Phase 1b multiple ascending-dose study of PRX002, an anti–alpha-synuclein monoclonal antibody, in patients with Parkinson’s disease [abstract]. Mov Disord. 2017; 32 (suppl 2). http://www.mdsabstracts.org/abstract/results-from-a-phase-1b-multiple-ascending-dose-study-of-prx002-an-anti-alpha-synuclein-monoclonal-antibody-in-patients-with-parkinsons-disease/. Accessed June 29, 2017. 21st International Congress of Parkinson’s Disease and Movement Disorders, June 4-8, 2017 Vancouver, BC. Abstract Number: 1418, Thursday, June 8, 2017.
6: Prothena Corporation PLC. Investor Presentation: Clinical Results from Phase 1b MAD Study of PRX002/RG7935 in Patients with Parkinson’s Disease. April 2, 2017.
7: Chen H, Burton EA, Ross GW, Huang X, Savica R, Abbott RD, Ascherio A, Caviness JN, Gao X, Gray KA, Hong JS, Kamel F, Jennings D, Kirshner A, Lawler C, Liu R, Miller GW, Nussbaum R, Peddada SD, Rick AC, Ritz B, Siderowf AD, Tanner CM, Tröster AI, Zhang J. Research on the premotor symptoms of Parkinson’s disease: clinical and etiological implications. Environ Health Perspect. 2013 Nov-Dec;121(11-12):1245-52. doi: 10.1289/ehp.1306967. Epub 2013 Aug 9. Review. PubMed PMID: 23933572; PubMed Central PMCID: PMC3855519.
8: Okura Y, Matsumoto Y. Development of anti-Abeta vaccination as a promising therapy for Alzheimer’s disease. Drug News Perspect. 2007 Jul-Aug;20(6):379-86. Review. PubMed PMID: 17925892.
9: Weihofen A, Patel H, Huy C, Liu C, Combaluzier I, Mueller-Steiner S, Cavegn N, Strobel L, Engber TM, Rhodes KJ, Hock C, Nitsch RM, Montrasio F, Grimm J, Dunah A, Weinreb PH. Human-derived α-synuclein antibody BIIB054 binds pathologic forms of α-synuclein and attenuates transmission of α-synuclein in vitro and in vivo [abstract]. Mov Disord. 2016; 31 (suppl 2). http://www.mdsabstracts.org/abstract/human-derived-synuclein-antibody-biib054-binds-pathologic-forms-of-synuclein-and-attenuates-transmission-of-synuclein-in-vitro-and-in-vivo/. Accessed May 28, 2017.
10: Weihofen A, Patel H, Huy C, Liu C, Combaluzier I, Mueller-Steiner S, Cavegn N, Strobel L, Kuznetsov G, Engber TM, Rhodes KJ, Hock C, Nitsch RM, Montrasio F, Grimm J, Hirst WD, Auluck PK, Dunah A, Weinreb PH. Binding and functional characterization of human-derived anti-alpha-synuclein antibody BIIB054. 13th International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD 2017), Vienna, Austria, 29-Mar-2017 11:00-13:00.
11: Brys M, Hung S, Fanning L, Penner NM, Yang M, David E, Fox Tt, Makh S, Graham D, Cedarbaum JM. Randomized, double-blind, placebo-controlled, single ascending dose study of anti-alpha-synuclein antibody biib054 in healthy volunteers. 13th International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD 2017), Vienna, Austria, 02-Apr-2017 11:45.
12: Chinta SJ, Lieu CA, Demaria M, Laberge RM, Campisi J, Andersen JK. Environmental stress, ageing and glial cell senescence: a novel mechanistic link to Parkinson’s disease? J Intern Med. 2013 May;273(5):429-36. doi: 10.1111/joim.12029. Review. PubMed PMID: 23600398; PubMed Central PMCID: PMC3633085.
13: Andersen JK. Senescence and the aging brain. Presentation at Rejuvenation Biotechnology 2014 (RB2014), August 21-23, 2014 Santa Clara, California. Parkinson’s Disease Session. Program p. 41. Video Presentation here.
14: Boominathan A, Vanhoozer S, Basisty N, Powers K, Crampton AL, Wang X, Friedricks N, Schilling B, Brand MD, O’Connor MS. Stable nuclear expression of ATP8 and ATP6 genes rescues a mtDNA Complex V null mutant. Nucleic Acids Res. 2016 Nov 2;44(19):9342-9357. Epub 2016 Sep 4. PubMed PMID: 27596602; PubMed Central PMCID: PMC5100594.



