Accumulation of soluble and insoluble aggregates of beta-amyloid protein (Aß) and other malformed proteins accumulate in brain aging and neurodegenerative disease, leading progressively to neuronal dysfunction and/or loss. These have long been widely accepted to be drivers of Alzheimer’s disease (AD) and other age-related dementias and neurological disorders such as Parkinson’s disease, and it has recently become increasingly clear that neuronal protein aggregates are the main driver of “normal” cognitive aging. To prevent and reverse the course of neurodegenerative disease and age-related cognitive dysfunction, the regenerative engineering solution is therapeutic clearance of extracellular aggregates (such as Aß plaques) and intracellular aggregates (such as soluble, oligomeric Aß).
Immunotherapeutic Aß clearance from the brain is a very active field of Alzheimer’s research, with at least seven passive, and several second-generation active, Aß vaccines currently in human clinical trials.(1) Of all Aß immunotherapies, he furthest advanced along the clinical pipeline are the passive monoclonal antibody vaccines bapineuzumab/AAB-001 (Janssen/Elan/Pfizer) and solanezumab/LY2062430 (Eli Lilly), both of which are currently in Phase III clinical trials. Other approaches, still in preclinical development, include the use of beta-amyloid-targeting affibodies, DNA and peptide vaccination targeting beta-amyloid epitopes, and catalytic cleavage of the beta-amyloid peptide itself. We now have a published report of preliminary findings from the first Phase I trial in an Aß-targeting vaccine with novel properties, and with the benefit of preliminary findings of outcomes that have only emerged with the experience of its forerunners in previous clinical trials.
Novel Target Antigen
The new contender — Hoffmann-La Roche/Morphosys candidate gantenerumab (R1450 or RO4909832) — is the first fully human anti-Aβ monoclonal antibody to enter clinical development: previous candidates have been humanized versions of murine antibodies, or derived from antibody fragments, or antibodies already present in pooled human immunoglobulin for injection (IVIgG). Gantenerumab was selected from a human phage display library and “optimized in vitro for binding with sub-nanomolar affinity to a conformational epitope expressed on amyloid-β fibrils … In peptide maps, both N-terminal and central portions of Aβ were recognized by gantenerumab.”(2) This, too, is a novel charcteristic of the new passive vaccine: the investigators claim it is unique amongst therapeutic antibodies, and certainly most anti-Aβ antibodies in clinical development recognize B-cell epitopes located either at the N-terminus of the protein (as does bapineuzumab) or a central span (eg. solanezumab); those that exploit T-cell targeted epitopes (including those elicited through active vaccination) map primarily to the central span and C-terminus of the peptide.(1)
Novel Clearance Mechanism
Gantenerumab may also be distinct from its passive vaccine competitors in using a cell-mediated mechanism of action for the removal of Aβ from the brain. AN1792, the first Aβ-targeting vaccine to enter clinical trials, was an active vaccine, and were mediated through T-cell responses specific to the carboxy terminal of the peptide; to date, passive antibodies have appeared to work by inducing efflux of Aβ from the brain, either through passage of the antibody through the blood-brain barrier (BBB) followed by sequestering of brain Aβ and then cotransportation of IgG-Aβ immune complexes back through the BBB (eg bapineuzumab), or through the “peripheral sink” mechanism of binding systemic Aβ and eliciting drawdown of soluble Aβ from behind it (eg. solanezumab). While still in preclinical development, the approach that SENS Foundation finds most exciting (on first principles, and based on results to date) is catalytic clearance of the beta-amyloid peptide itself.*
In contrast to all of these, gantenerumab, appears to be the first passive vaccine to stimulate microglial phagocytosis of Aβ. “In functional assays gantenerumab induced cellular phagocytosis of human amyloid-β deposits in AD brain slices when co-cultured with primary human macrophages”;(2) “In ex vivo studies of human brain slices from an independent sample of patients who had AD … Gantenerumab induced phagocytosis of human amyloid in a dose-dependent manner ex vivo.”(3) Promisingly, and consistent with such a mechanism, when tested model AD mice bearing transgenic familial AD mutations in amyloid precursor protein and presenillin-2, “gantenerumab showed sustained binding to cerebral amyloid-β and, upon chronic [5 mo] treatment, significantly reduced small amyloid-β plaques by recruiting microglia and prevented new plaque formation. Unlike other Aβ antibodies, gantenerumab did not alter plasma Aβ, suggesting undisturbed systemic clearance of soluble Aβ.”(2)
Those findings have now been supplemented with the first data derived from human clinical trials.
Phase I Clinical Trial Data
In a Phase I clinical trial,(3) 18 mild to moderate AD patients, aged 50-90 y, recruited from 3 university medical centers, were randomized to receive 7 monthly intravenous infusions of placebo or gantenerumab (60 or 200 mg). These doses were derived from previous, unpublished, single- and multiple-dose Phase I studies. In practice, however, while all subjects receiving the lower dose of antibody received the full course of therapy, few patients in the higher-dose group received all 7 infusions, due to early termination (see below): “One patient received 2 infusions, 2 patients received 3 infusions, 2 patients received 4 infusions, and 1 patient received 5 infusions.”(3) Sixteen of these patients underwent carbon 11–labeled positron emission tomographic imaging with the Aβ-binding imaging agent, Pittsburgh Compound B (PiB-PET).
The results: based on PiB-PET, “The mean (95% CI) percent change from baseline difference relative to placebo (n = 4) in cortical brain amyloid level was -15.6% (95% CI, -42.7 to 11.6) for the 60-mg group (n = 6) and -35.7% (95% CI, -63.5 to -7.9) for the 200-mg group (n = 6).”(3) (See Figures 3 and 4, reproduced from original report, below). The approximate doubling of brain Aβ clearance in the higher-dose group is all the more remarkable considering the truncated course of therapy experienced by most (see below), and appears on a nominal basis to represent a faster rate of clearance than that previously reported for bapineuzumab over the course of 18 months (and that, without clear evidence of a dose-proportionate response).(4)
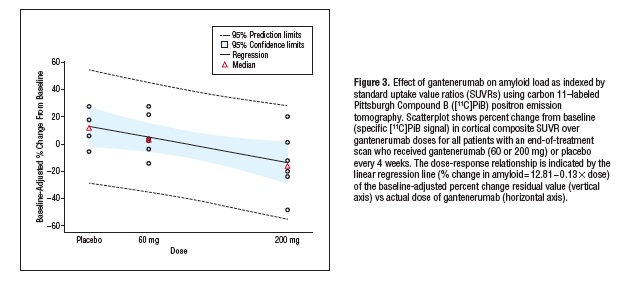
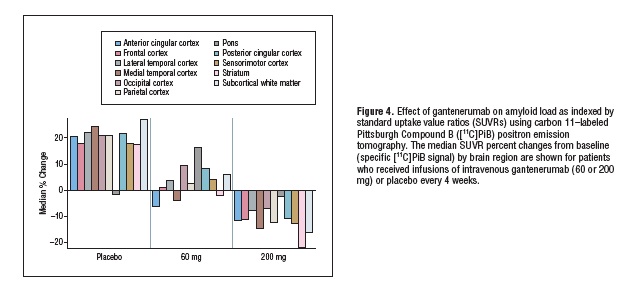
Reproduced from (4). Copyright American Academy of Neurology and the Authors.
The reason for the early termination of the trial was — unfortunately but not unexpectedly — the appearance of an adverse reaction in the high-dose gantenerumab group: MRI revealed that 2 subjects underwent transient periods of focal cerebrovascular inflammation or vasogenic oedema, coinciding with sites with the greatest local clearance of Aβ deposits.(3) Their appearance, while not welcome, would have been anticipated, based on their earlier occurrence in Phase II trials with bapineuzumab(4) and possibly solanezumab,(5) but ongoing study of these “amyloid-related imaging abnomralities” (ARIA)(11) now suggests that they are less concerning than they had initially appeared. Data from cerebral imaging studies of the general, non-AD population of Rotterdam show that haemosiderin abnormalities (ARIA-H) consistent with cerebral microbleeds occur in 3-15% of older adults,(6) and several studies presented at the 2011 Alzheimer’s Association International Conference (AAIC, formerly ICAD) suggest that vasogenic oedema (ARIA-E) may also be common in untreated subjects with the disease. In fact, reports there and elsewhere (including preclinical studies on the mechanism of such abnormalities, and a central blinded review of sequential brain images from the Phase II bapineuzumab trial) suggest that their increased occurrence in patients treated with Aβ-binding therapeutic antibodies is not only relatively mild and transient, but a potentially positive rough indicator of successful mobilization of brain Aβ.*
The combination of initial, dose-dependent reduction of brain Aβ as detected on PiB-PET, plus (ironically) the occurrence of vasogenic oedema at locations adjacent to the sites where clearance is greatest, is tantalizing evidence that gantenerumab is efficacious in removing these malformed proteins from the brain. The study was too small and early-phase to permit assessment of cognitive outcomes, but with the success of the Phase I trial, a Phase IIb clinical trial is now underway, which will test the effects of subcutaneous gantenerumab (225 or 105 mg every 4 weeks for 104 weeks) vs. placebo on cognitive and functional outcomes in subjects with prodromal AD (identified based on partner-observed, gradual reductions in memory and cerebrospinal fluid biomarkers, but without dementia (Mini-Mental State Exam score ≥24).
The Race is On
With promising preliminary human data and what appears to be a mechanism of Aβ clearance, the advancement of gantenerumab into preliminary efficacy testing places one more damage-removal therapeutic into the race for disease-modifying therapies for AD. Should it ultimately be successful and its migroglial cell-mediated mechanism of action be validated, it would have the advantage of being potentially in synergy with lysosomal fortification with novel hydrolases to enhance microglial lysosomal hydrolysis of engulfed Aβ.
Rejuvenation biotechnology is the application of the principles of regenerative medicine to the damage to cellular and molecular structures that accumulate in aging tissues — the structural damage that disables those structures’ function and leads to loss of homeostasis and the progressive rise in frailty, disease, disability, and death that people now suffer with age. Because the damage is multifarious, a platform of rejuvenation biotechnologies, rather than a single, all-encompassing “youth pill,” will be required to achieve the robust rejuvenation of aging humans, restoring youthful health and vitality.
SENS is a strategy for engineering negligible senescence, based on this heuristic — not a prescriptive list of therapies in development whereby it shall be executed. The rapidly-expanding group of agents, each with a meaningfully-distinct mechanism of Aβ clearance, entering into the clinical pipeline and in increasingly advanced stages of human clinical testing for the arrest and reversal of Alzheimer’s disease, bodes well for the early achievement of the first rejuvenation biotechnology. SENS Foundation is proud to be engaged in its mission of catalyzing the progress toward a mature rejuvenation biotechnology industry; with that proof of concept, the wider biomedical field should become more alive to the application of the damage-removal heuristic to the many different kinds of aging damage underlying age-related disease. The engagement of a wide range of scientists in academia and industry, and aggressive funding of rejuvenation research, will accelerate progress toward a comprehensive panel of rejuvenation biotechnologies, and the achievement of thoroughgoing biomedical restoration of youthful health, vigor, and longevity.
* The theoretical advantages of catalytic antibodies as a mechanism for the therapeutic clearance of malformed protein deposits, combined with promising results to date in preclinical studies using such antibodies to clear brain Aβ, were key considerations leading to SENS Foundation’s funding of research into catalytic antibodies for the removal of senile cardiac amyloidosis.
References
1: Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010 Feb;6(2):108-19. Review. Erratum in: Nat Rev Neurol. 2010 Apr;6(4):183. PubMed PMID: 20140000; PubMed Central PMCID: PMC2864089.
2: Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, Messer J, Oroszlan K, Rauchenberger R, Richter WF, Rothe C, Urban M, Bardroff M, Winter M, Nordstedt C, Loetscher H. Gantenerumab: A Novel Human Anti-Aβ Antibody Demonstrates Sustained Cerebral Amyloid-β Binding and Elicits Cell-Mediated Removal of Human Amyloid-β. J Alzheimers Dis. 2011 Sep 28. [Epub ahead of print] PubMed PMID: 21955818.
3 : Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, Klunk WE, Ashford E, Yoo K, Xu ZX, Loetscher H, Santarelli L. Mechanism of Amyloid Removal in Patients With Alzheimer Disease Treated With Gantenerumab. Arch Neurol. 2011 Oct 10. [Epub ahead of print] PubMed PMID: 21987394.
4 : Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Rodriguez Martinez de Liano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010 Apr;9(4):363-72. Epub 2010 Feb 26. PubMed PMID: 20189881.
5: Bonetta L. Paris: Renamed ARIA, Vasogenic Edema Common to Anti-Amyloid Therapy. Alzforum (online resource). 2011 Jul 29. Accessed 2011-09-28.
6: Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, Vernooij MW. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011 Mar;42(3):656-61. Epub 2011 Feb 9. PubMed PMID: 21307170.
7: Zago W, Kinney G, Schroeter S, Khan K, Games D. Microvascular changes associated with passive immunotherapy in PDAPP mice – Potential implication for the etiology of vasogenic edema. Alzheimer’s Association International Conference, Paris. 2011 Jul 16-21. Abstract P3-052.
8: Sperling R, et al Revised estimates of incidence and risk factors for amyloid related imaging abnormalities (ARIA) in the phase 2 studies of bapineuzumab for mild to moderate Alzheimer’s disease. Alzheimer’s Association International Conference, Paris. 2011 Jul 16-21. Abstract P4-438.
9: Salloway S, Sperling R, Honig, L Arrighi M, Wei H-L, Yuen E, Liu E, Morris K, Grundman M, Brashear R. Long-term follow-up of AD patients treated with bapineuzumab in phase 2. Alzheimer’s Association International Conference, Paris. 2011 Jul 16-21. Abstract O4-08-07.
10: Salloway S. Vasogenic edema in immunotherapy: Sign of efficacy or danger? Alzheimer’s Association International Conference, Paris. 2011 Jul 16-21. Abstract S5-01-05
11: Sperling RA, Jack CR Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, Black RS, Brashear HR, Grundman M, Siemers ER, Feldman HH, Schindler RJ. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011 Jul;7(4):367-85. PubMed PMID: 21784348.



