Science has always been a great joy of mine for as long as I can remember. As a student pursuing a degree in biochemistry through the chemistry department at the University of California Santa Barbara (UCSB), I have worked as an undergraduate researcher for two years in the lab of Dr. Alison Butler studying mechanistic bioinorganic chemistry, metallobiochemistry, and chemical biology.
My work at UCSB revolves around the ability of microorganisms to harvest iron from their environment using small molecules termed siderophores—these siderophores are capable of scavenging and binding to iron with incredible strength in order to bring this essential nutrient back to the cell. As an undergraduate researcher in Dr. Butler’s lab, I have worked with the marine bacterium Vibrio harveyi and the siderophore amphi-enterobactin (discovered in Dr. Butler’s lab1) which this bacterium synthesizes. My project has been focused on transforming potential siderophore-producing genes from V. harveyi into E. coli in order to over-express the siderophore. Successful transformation and siderophore isolation would both identify the gene which produces amphi-enterobactin and reveal the details of amphi-enterobactin’s chemical properties and mechanism. I have also done work using organic synthesis to create derivatives of the siderophore cyclic trichrysobactin in Dr. Butler’s lab in order to explore the potential application of these molecules for wet adhesion purposes in both industrial and medicinal fields2. The synthesis of these molecules involves the creation of symmetrical peptide linkers around a tris(2-aminoethyl)amine (TREN) core so that the binding proteins in mussel foot byssal plaques can be synthetically optimized for industrial and/or medicinal adhesion purposes. As seen in mussel foot byssal plaques, charged lysine or arginine residues use charge-charge repulsion to sweep away the positively charged hydration layer which forms around submerged objects3. Then, two hydroxyl groups of a DOPA amino acid residue (known as a catechol group) allow bidentate hydrogen bonding to the previously inaccessible, submerged surface. There is also some evidence of metal chelation by closely associated carbonyl groups in the peptide backbone3. The adhesive properties of these molecules can be assessed using a Surface Forces Apparatus (SFA), which measures the repulsive and attractive forces between two surfaces. Both of these projects remain active in Santa Barbara.
Solubilizing Harmful Cholesterol and Oxysterols for the Treatment of Atherosclerotic Plaques
Here at the SENS Research Foundation, I have been working with derivatives of drugs which have been shown to solubilize cholesterol and/or harmful derivatives of cholesterol such as oxysterols. Cholesterol and its harmful derivatives are taken up by cells as they attempt to process these molecules for the body. As the macrophage lysosomes (small organelles for degrading waste) become more and more saturated with debris from HDL (high density lipoproteins— “good” cholesterol) and LDL (low density lipoproteins— “bad” cholesterol) molecules, the cells become useless and form foam cells when they can no longer process the excess cholesterol. These malfunctioning foam cells accumulate in the arterial walls, becoming part of the problem instead of the solution and contributing to the plaques which cause atherosclerosis, or hardening of the arteries.
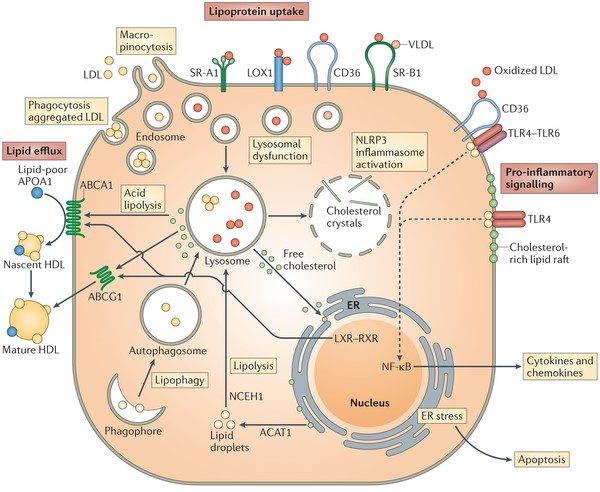
Figure 1. Diagram of a macrophage becoming a foam cell.
Diagram of a macrophage becoming a foam cell as it uptakes and attempts to process lipoproteins, recruits cytokines, and signals inflammation at the point of plaque formation.—Moore, K., Sheedy, F., Fisher, E. (2013). Macrophages in Atherosclerosis: A Dynamic Balance. Nature Reviews Immunology.
With more than three million cases of atherosclerosis per year, this disease contributes significantly to heart disease, widely known as the number one killer in the United States. In order to develop a safer, more effective treatment for atherosclerosis, a rational drug design venture is underway at SENS. Various tests have been and will be conducted with novel drugs for the purpose of removing cholesterol and its derivatives from atherosclerotic plaques. For my project, in vitro, ex vivo, and in silico experiments have been designed to assess the effectiveness and safety of these drugs for the purpose of reducing atherosclerotic plaques in human arteries.
Future Plans:
I plan to eventually pursue a PhD in biochemistry and apply this doctorate to the field of education, pharmaceuticals, and/or environmental sustainability. I would like to keep my career research-oriented and I hope to someday make my mark on the world of science. The exciting world of scientific discovery has fueled my motivation to put in the hard work which I know it will require of me, and that is what makes it worth every drop of blood, sweat, and tears.
References:
1. Zane, H., Naka, H., Rosconi, F., Sandy, M., Haygood, M., Butler, A. (2014). Biosynthesis of Amphi-enterobactin Siderophores by Vibrio harveyi BAA-116: Identification of a Bifunctional Nonribosomal Peptide Synthetase Condensation Domain. J. Am. Chem. Soc. 136(15):5615-5618.
2. Sandy M, Butler A. 2011. Chrysobactin siderophores produced by Dickeya chrysanthemi EC16.. J Nat Prod. 74(5):1207-12.
3. Maier GP, Rapp MV, J. Waite H, Israelachvili JN, Butler A. 2015. Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science. 349(6248):628-32I.
4. Yu, J., Y. Kan, M. Rapp, E. Danner, W. Wei, S. Das, D. R. Miller, Y. Chen, J. H. Waite, and J. N. Israelachvili. (2013) Adaptive Hydrophobic and Hydrophilic Interactions of Mussel Foot Proteins with Organic Thin Films. Proceedings of the National Academy of Sciences 110(39):27450-27455.


