My name is Wenyi Zhu, and I’m entering my 4th year at Ernest Mario School of Pharmacy at Rutgers University. This summer, I will be working in Dr. Evan Snyder’s lab at the Sanford Consortium for Regenerative Medicine. Under the mentorship of Dr. Lina Mastrangelo, I will be investigating the effects of LRRK2 protein and PARK1 mutations on autophagy in the hopes of understanding more about the progression of Parkinson’s disease.
Over the past summer, I was a fellow in the Rutgers Summer Undergraduate Research Fellowship program. Under the tutelage of Dr. Jason R. Richardson, I studied the effects of environmental toxicants on beta amyloid precursor protein levels in cultured neuroblastoma cells through immunofluorescence imaging. Beta amyloid precursor protein (APP) is cleaved to beta amyloid peptides, which contribute to form the distinctive extracellular amyloid plaques that are one of the hallmarks of Alzheimer’s disease (AD). The environmental toxicants that I investigated were DDT (dichlorodiphenyltrichloroethane), a pesticide that was widely used before being banned in the U.S., and lead acetate, a potent neurotoxin. Both DDT and lead acetate reached their peak usage during the childhood of the Baby Boomer generation – the same generation that are currently most at risk for AD. Since there are both genetic and environmental risk factors that make a person predisposed to AD, I was interested to see what effect DDT and lead would have on AD substrates. Previous work from the Richardson lab showed that levels of DDE, a metabolite of DDT, were higher in serum levels of Alzheimer’s patients as compared to the control group1. Likewise, the Zawia lab showed that cells that have been exposed to higher concentrations of lead had higher levels of APP concentration as opposed to the control group2.
My project examined the levels of APP in cells that have been treated with DDT and lead acetate, both individually as well as a combined treatment. My preliminary data showed that there are changes in APP levels. However, further testing will be needed to confirm these initial results. Therefore, I plan to continue this project through the Ernest Mario School of Pharmacy Honors Program and will be working towards completing my honors thesis on the effects of these environmental toxicants and APP levels.
Exploring the Role of LRRK2 and PARK1 Mutations on Autophagy
Under the guidance of Dr. Lina Mastrangelo, my SRF Summer Scholars Project will determine how certain gene mutations detected in Parkinson’s disease patients affect autophagy in induced pluripotent stem cells (iPSCs). Parkinson’s disease (PD) is an age-related neurodegenerative disease that is characterized by the loss of dopaminergic cells in the substantia nigra (the area of the brain that controls motor function) and the formation of abnormal intracellular protein aggregates called Lewy bodies that disrupt normal neuronal function. Patients affected by PD exhibit primary motor impairment, such as resting tremors, bradykinesia (slow movement), and postural instability3. PD can manifest in 2 forms: the more common sporadic form as well as the familial form, where specific mutations in inherited genes make an individual more susceptible to getting PD. Both the leucine-rich repeat kinase 2 (LRRK2) protein and the Parkinson’s disease 1 (PARK1) genes have been identified to be risk factors for familial Parkinson’s4.
For this study, I will use induced pluripotent stem cells (iPSCs) derived from the skin of a Parkinson’s patient. iPSCs are unique in that they have the potential to be differentiated into various types of cells, ranging from muscle to liver to blood cells. In this case, the iPSCs will be differentiated into neurons and astrocytes. Astrocytes are another type of cells found in the brain and primarily act as a support structure for neuronal migration and differentiation. There are two different iPSCs lines – one that carries the LRRK2 mutation (Figure 1) and one that carries the PARK1 mutation. Mutations in both the LRRK2 and the PARK1 genes are known to be risk factors of PD but function through different molecular pathways. The PARK1 gene mutation is most commonly associated with early onset PD. Patients with the PARK1 mutation show an increase in levels of α-synuclein protein, which is the main component of intracellular Lewy bodies. On the other hand, the LRRK2 mutation is generally associated with late onset PD and is believed to alter downstream signaling pathways in neurons5. While the affected genes have been identified, the effects of the mutation on cellular function remains unclear. Therefore, my Summer Scholars project will explore the role of these proteins in autophagy, which is one of the pathways proposed to play a role in PD progression.
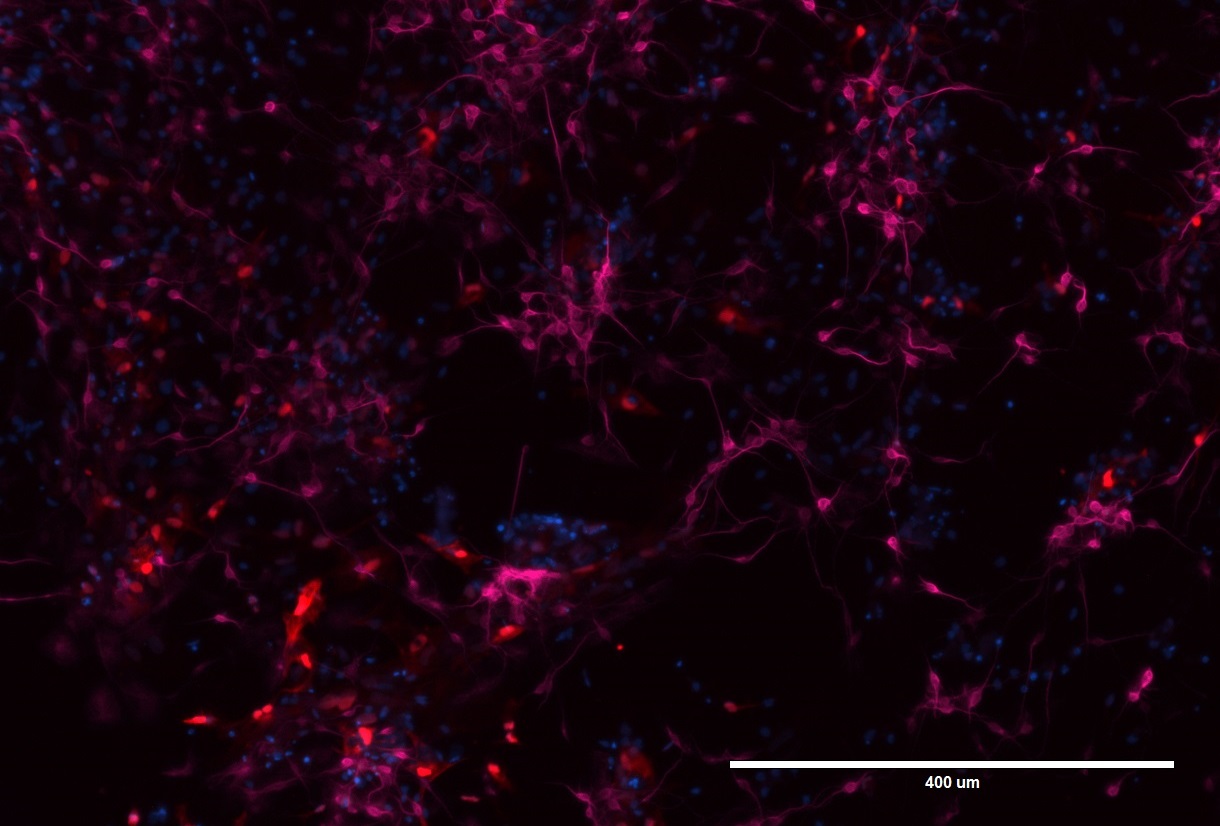
Figure 1. LRRK2 neurons and astrocytes stained for MAP2 and GFAP.
Pictured here are neuronal cells and astrocytes that have been differentiated from iPSCs that carry the LRRK2 mutation. The neurons have been stained with MAP2 (microtubule-associated protein 2; pink), which is a major cytoskeletal protein involved in the microtubule assembly, a key pathway for neurogenesis. GFAP (glial fibrillary acidic protein; red) is a specific marker for astrocytes – one of the types of glial cells in the nervous system. DAPI (blue) is a marker for DNA and marks the nucleus.
Autophagy is the cell’s natural defensive mechanism – a way for a cell to get rid of toxic buildup of protein either due to normal cellular function or different environmental stressors. The main organelle responsible for autophagy is the lysosome – an intracellular organelle that possesses a highly acidic interior which facilitates breakdown of proteins and other peptides. There are various types of autophagy – non-specific macroautophagy, where proteins are sequestered into vesicles and fused to lysosomes for degradation and the more specific chaperone-mediated autophagy, where chaperone proteins bind to specific substrates and transport them to the lysosome for degradation6. It is believed that mutations in the LRRK2 protein and PARK1 genes inhibit or hinder the cell’s ability to mediate autophagy, thus leading to the accumulation of toxic Lewy bodies inside the neuron and ultimately to Parkinson’s disease.
Through western blot analysis and various imaging techniques, I will measure the presence and concentration of different autophagy substrates in cells carrying the LRRK2 and PARK1 mutations to determine what kind of affect they would have on autophagy and whether a combination of these mutations would produce a synergistic effect.
Future Plans:
In the fall, I plan on returning to school to complete my degree and to finish my honors thesis. While I am not completely certain what career path I will take after I graduate, I know that I want to continue to work in the neurology field, either by pursuing a post-graduate residency to enter the clinical route or work in the pharmaceutical industry to focus on drug development for neurodegenerative diseases.
References:
1. Richardson JR, Roy A, Shalat SL, et al. Elevated Serum Pesticide Levels and Risk for Alzheimer Disease. JAMA neurology. 2014;71(3):284-290. doi:10.1001/jamaneurol.2013.6030.
2. Huang H, Bihaqi SW, Cui L, Zawia NH. In vitro Pb exposure disturbs the balance between Aβ production and elimination: the role of AβPP and Neprilysin. Neurotoxicology. 2011;32(3):300-306. doi:10.1016/j.neuro.2011.02.001.
3. The Michael J. Fox Foundation. “Parkinson’s Disease Causes | Parkinson’s Disease Information.” N.p., n.d. Web. 20 May 2016
4. Klein C & Westenberger A (2012). Genetics of Parkinson’s Disease. Cold Spring Harbor Perspectives in Medicine, 2(1), a008888. http://doi.org/10.1101/cshperspect.a008888
5. Cookson MR (2010). The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nature Reviews. Neuroscience, 11(12), 791–797. http://doi.org/10.1038/nrn2935
6. Cuervo AM, & Wong E (2014). Chaperone-mediated autophagy: roles in disease and aging. Cell Research, 24(1), 92–104. http://doi.org/10.1038/cr.2013.153


