My name is Ryan Leung from New Bedford, MA. I am a recent graduate of Tufts University, where I received my BS in Biomedical Engineering. Over my time in various research environments, I developed a passion for translational science and regenerative medicine.
My interest began in my first and second years at Tufts while working at the Sackler School of Biomedical Sciences under the guidance of Dr. Jonathan Garlick. There, my project leveraged 3D human skin equivalents to examine impaired wound healing in diabetics. My work in particular focused on the characterization of patient-derived diabetic skin models and the examination of these skin models in response to simulated wounding. During my time, I identified various genetic, epigenetic, and proteomic biomarkers that may predispose healing impairment and foot ulcer development in patients who suffer from diabetes. By gaining a greater understanding of the physiological mechanisms of impaired wound healing, Dr. Garlick hopes we will be able to develop more effective therapies and standards of care.
This experience sparked my interest in regenerative medicine and encouraged my decision to join the Tissue Engineering Resource Center in my third year at Tufts. Under the mentorship of Dr. David Kaplan over the past two years, I worked to engineer a complex 3D model of human kidney tissue for disease modeling and drug screening. My approach featured a novel biomaterial scaffold that combined the biocompatibility and tunable mechanical properties of silk proteins with the cell signaling and complexity benefits of extracellular matrix molecules. The resulting tissue platform is capable of sustaining long-term 3D tissue culture of renal endothelial and epithelial cells in vitro and offers more physiologically-relevant structure and function than many existing models. Platforms such as this are capable of more accurately recapitulating the in vivo disease environment and will allow more clinically-relevant therapies to emerge.
In addition to my laboratory research projects, I also participated in a practical internship at Thermo Fisher Scientific. As a member of the Cell Biology R&D team, I validated a cell culture media product and executed proof-of-concept experiments for a novel cell culture platform. Moreover, I learned how to identify needs in biological research and how to design, test, and re-test a product before bringing it to market.
Bioartificial Ovary: Generation of Functional Ovarian Follicles for Infertility Treatment
This summer, I am working at the Wake Forest Institute for Regenerative Medicine under the supervision of Dr. John Jackson and Dr. James Yoo. My project aims to develop a functional bioartificial ovary capable of serving as a cell-based therapy for women who suffer from compromised ovarian function and disease-induced infertility.
Impaired fecundity (the inability to have a child) affects approximately 6.7 million women in the U.S. — about 11% of the reproductive-age population.[1] While most common cases can be treated with conventional therapies such as medication (pharmaceuticals that supplement hormone production and stimulate ovulation) or surgery (intrauterine insemination or hysteroscopic surgery), there are many cases where these options are not viable. For example, disorders, such as polycystic ovary syndrome and premature ovarian failure, as well as cancer treatments, such as chemo- and radiation-therapy, can lead to infertility and result in cases where typical treatment methods are either too dangerous to apply or are incapable of functioning properly.[2,3] Furthermore, these conventional therapies are not without risk, as they may increase the likelihood of breast cancer, stroke, blood clot formation, and heart disease.[4,5]
A potential solution for these patients is the cryopreservation of ovarian tissue containing immature follicles or the cryopreservation of immature follicles isolated from ovarian tissue. By extracting tissue instead of oocytes themselves, the required, and potentially harmful, hormone injections are avoided. Additionally, ovarian tissue can be obtained from the patient at the time of diagnosis, eliminating the need for any additional surgical procedures. The follicles acquired from the resulting tissue can then be developed in vitro to produce oocytes capable of being fertilized for use as an infertility therapy.
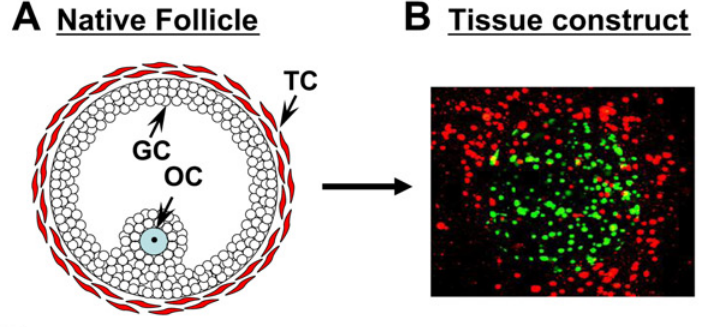
Figure 1. Structure of an Ovarian Follicle.
A) The cellular structure of a native ovarian follicle [GC= granulosa cell; TC= theca cell, OC= oocyte]. B) Fluorescent image of an early tissue engineered construct mimicking the native structure and arrangement of an ovarian follicle. Granulosa cells (green) in the middle of the construct surrounded by Theca cells (red). The current project aims to build upon this model by also generating and identifying a viable and mature oocyte.
The project at-hand aims to investigate this solution by fabricating a functional ovarian construct capable of folliculogenesis (the development process of an ovarian follicle) and mature oocyte formation. Specifically, the tissue platform I will be developing will combine primary ovarian cells with a 3D collagen-based scaffold to create a construct that closely mimics the native environment and structure of the ovary. The hope is that the ovarian construct will yield mature oocytes that can be isolated and further assessed for their viability as an infertility treatment.
Future Plans:
References:
[1] Chandra A, Copen CE, Stephen EH, Infertility and impaired fecundity in the United States, 1982-2010: data from the national survey of family growth. Nat Health Stat Rep. 2013; 67.
[2] Nieman CL, Kazer R, Brannigan RE, Zoloth LS, Chase-Lansdale PL, et al. Cancer survivors and infertility: a review of a new problem and novel answers. J Support Oncol. 2006; 4:171–78.
[3] Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009; 360:902–11.
[4] Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006; 21:2583–92.
[5] Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001; 7:535–43.


