My name is Celine-Lea Halioua-Haubold, and I am a rising senior studying Neuroscience at the University of Texas (UT) at Austin. I have re-joined Dr. Evan Snyder’s lab at the Sanford Burnham Prebys Medical Discovery Institute as a 2016 SRF Summer Scholar to continue my 2015 Summer Scholar project. I have long known my future career will lie somewhere in the field of Neuroscience, but only due to my internship last summer did I realize my passion lies in research and development of therapeutics for neurological diseases. A large number of the diseases that plague the central and peripheral nervous system lack an effective treatment, never mind a cure. As millions suffer from these types of diseases, there is a massive need for driven, innovative research in this area. Through the SRF program, I have begun to dip my toes into this field.
My first experience with wet-lab research was in 2013 through the Freshman Research Initiative (FRI) at UT, which places second-semester freshman in productive labs across multiple departments. I worked in the Nanomaterials for Chemical Catalysis FRI lab, led by Dr. Keith Stevenson. In 2014, I became an FRI mentor for incoming students. As a mentor I assisted teaching the relevant lab theory and basic bench chemistry techniques, along with ensuring safety in the lab. The majority of my research experience has come from working as a research assistant in Dr. Richard Morrisett’s lab from 2014 to 2015, where I worked under the supervision of Dr. Regina Mangieri. The lab investigates the neuronal changes induced by and associated with alcohol dependency. After assisting Dr. Mangieri for half a year, I began my own project investigating DNA-templated silver nanoclusters as a potential novel in vivo cell tracer. With the assistance of Dr. Mangieri, I provisionally showed that the Ag-NCs were non-toxic, stable in vivo for many weeks, had long-lasting bright fluorescence, and successfully labeled neuronal cell bodies.
Between 2015 and 2016, I studied abroad at Uppsala Universitet in Uppsala, Sweden, where I loosely followed the Bio- and Nanomaterials master’s program courses in the Department of Chemistry – Ångström. I briefly was involved with two research projects in Ångstrom. Under Dr. Sara Gallineti, I worked on a project investigating methods of strengthening calcium phosphate cements for eventual use as a bone cement on patients whom have broken a bone. I also assisted Maruthibabu Paidikondala with his project investigating a novel method of delivering DNA into cells using hyaluroran, a molecule which is part of the extracellular matrix, the structural and biological support secreted by cells to support surrounding cells.
Last summer, I investigated cholera subunit-B as a method of delivering therapeutics to the quickest and deadliest type of brain cancer, glioblastoma (Bleeker et al., 2012). Cholera Toxin Subunit B (CTB) is the protein which is part of the toxin released by V. cholerae. Infections of patients with this bacterium manifests as the disease cholera. CTB itself is non-toxic and interesting to researchers due to its neuronal specificity and efficacy in entering cells (Bharati et al, 2016; Harris et al., 2013). I have provisionally shown that by attaching a specific protein to CTB, I could sensitize these cancer cells to a secondary treatment, causing earlier cell death (Figure 1). Data collected also suggested that CTB enters the cell very quickly and does not degrade nor get pushed out of the cell – a fate that befalls many cell-targeting treatments due to the cell’s natural protective measures. Although these pilot experiments will require further study, the analysis suggests the potential of CTB as a therapeutic delivery method.
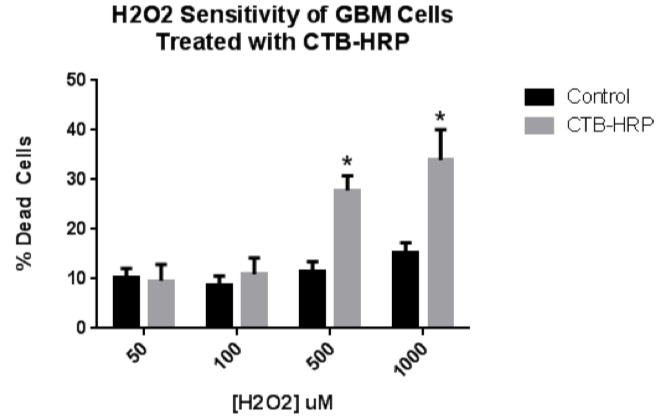
Figure 1. H2O2 sensitivity of CTB-HRP-treated GBM cells.
To investigate if a CTB-delivered protein could sensitize GBM cells to a secondary treatment, GBM cells were incubated with CTB-HRP and then exposed to oxidative stress. Cells were incubated with 2 ug/mL of CTB-HRP and then treated with either 50, 100, 500, or 1000 uM hydrogen peroxide diluted into serum-free medium. Cell viability was assessed in experimental and control wells after 24 hours. No statistical difference was observed between the 50 and 100 uM treatments and the control. However, the 500 and 1000 uM treatments produced a statistically-significant higher level of cell death in response to the peroxide treatment.
Outside of research, I am interested in Germanic languages, having studied Swedish and German and plan to begin Dutch in any free time found. Additionally, I have ridden horses for over 14 years and compete regularly in the regional dressage circuit in Texas.
Cholera Subunit-B Mediated Therapeutic Delivery to the Enteric Nervous System
While the results last summer were promising, Dr. Snyder and I are moving on to investigating the potential of CTB-mediated therapy of the enteric nervous system (ENS), the neuronal system innervating the gastrointestinal (GI) tract (Thapar, 2007), due to the inherent benefits of using the cholera protein on the system it was evolutionarily designed to target. I plan to take advantage of the native ENS targeting of CTB. This new experimental design will provide a better system in which to test the viability of CTB-mediated therapeutics delivery.
The primary goal of the proposed research is to determine if Cholera Toxin B is a viable candidate for delivering secondary proteins to serve therapeutic purposes in neuronal cells. I have designed a simplified proof-of-concept set of experiments in order to support this hypothesis. To model a pathological cellular state, I will expose my cells to oxidative stress, which when left untreated eventually causes cell death. I will then attempt to either rescue or protect my cells from this oxidative stress with antioxidant enzymes delivered by CTB. Antioxidant enzymes are regularly produced by the cell to combat biologically natural levels of oxidative stress but not always in sufficient quantities to rescue the cells from in vitro-induced oxidative stress (Birben et al., 2012). If CTB successfully delivers these antioxidant enzymes into the cell, the treated cells should show lower rates of cell death in comparison to the untreated cells.
If this pilot experiment is successful, it will be the first step to eventually developing treatments for a multitude of enteric neuron maladies, including neuropathy, the premature death of neurons, and neuroinflammation, the inflammation of neurons caused by irritation. Enteric neuropathy is common in diseases including diabetes mellitus (Forgacs et al, 2015) and Parkinson’s (Braak et al, 2006), and neuroinflammation (Lakhan et al, 2010) is seen in a multitude of diseases. Irritation and damage of the enteric neurons causes enduring GI distress for the patient. These symptoms can be crippling, and currently there is no targeted treatment for the underlying cause of the GI distress – only “Band-Aid” treatments, such as pain medication and anti-inflammatories (Rudman, 2013). Therefore, should CTB-mediated targeted therapeutic delivery to the enteric system be successful, the benefit to patients is undeniable.
Future Plans:
I hope to study medicine after completing my neuroscience degree. I plan to study medicine in Europe, likely the Netherlands, due to their research-centered medical programs along with my interest in Northern Europe. In the long-term, I am interested in becoming involved in either the academic or industrial sector, assisting in the research and development of drugs to treat neurological disease. I believe it is very important to encourage and foster international scientific cooperation and collaboration, and hope to assist in this endeavor as my career progresses.
References:
Bharati, K., & Ganguly, N. K. Cholera toxin : A paradigm of a multifunctional protein Indian J. Res. Med. 2016;133(2):179–187.
Birben, E. et al. Oxidative Stress and Antioxidant Defense. WAO Journal. 2012;5:9-19.
Bleeker, Fonnet E.; Molenaar, Remco J.; Leenstra, Sieger. Recent advances in the molecular understanding of glioblastoma”. Journal of Neuro-Oncology. 2012;108(1):11–27
Braak, H., de Vos, RA., Bohl, J., and Del Tredici, K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neuroscience Letters. 2006;396(1):67-74.
Forgacs I, Raja O. Diabetes and the gastrointestinal tract. Medicine. 2015;43:347-351.
Harris, J. B., Larocque, R. C., Qadri, F., Ryan, E. T., & Calderwood, S. B. Cholera. Lancet. 2016;379:2466–2476.
Lakhan S, Kirchgessner A. Neuroinflammation in inflammatory bowel disease. Journal of Neuroinflammation. 2010;7:37-37.
Rudman, D. On-target and Off-target based-based Toxicologic Effects. Toxic Pathology. 2013;41:310-314.
Thapar, N. Future horizons in the treatment of enteric neuropathies. Journal of Pediatric Gastroenterology and Nutrition. 2007;45:S110–114.


