Growing up in West Milford, New Jersey, I had very limited exposure and opportunities to learn about research first-hand. During my time at Purdue University, I earned a B.S. in biochemistry and minors in both French and entrepreneurship and had the opportunity to participate in some research for the first time. During my undergraduate studies, my most significant research experience was work in Dr. James Forney’s lab in the Department of Biochemistry characterizing the effects of C-terminal mutations of the Small Ubiquitin-like Modifier (SUMO) protein in Tetrahymena thermophila, a single-celled protozoan organism.
SUMO is a conserved protein amongst most eukaryotic organisms, and it regulates many different cellular processes in its attachment to substrates. In humans, deficient attachment of SUMO to substrates has been linked to heart disease and neurodegenerative disorders. The goal of my research was to generate and characterize mutants of SUMO in Tetrahymena to find mutants that are unable to attach to substrates, or are unable to be sumoylated themselves, with the hopes of characterizing a novel phenotype or identifying novel substrates. I discovered that altering a specific motif of SUMO greatly decreases its stability and its ability to attach to target proteins. I received a grant from the American Society for Biochemistry and Molecular Biology (ASBMB) to present these findings at the 2015 Experimental Biology conference.
My research experiences at Purdue sparked a passion for me to continue to pursue different research areas. When I received a link to apply for the SENS Research Foundation Summer Scholar Program from my advisor, I knew that the SRF Summer Scholars Program would provide me with an amazing opportunity to do some translational regenerative medical research, a field that had always been of interest to me since I began my undergraduate research.
Engineering Ovarian Follicles in vitro in a 3-D Collagen Matrix
This summer, I will be working in Dr. Anthony Atala and Dr. James Yoo’s lab under Drs. Myung Jae Jeon and Young Sik Choi studying ovarian cell therapies that will be able to produce natural levels of sex steroids that can be controlled by feedback mechanisms and, hopefully, produce viable oocytes. The importance of this research is providing effective therapies for hormone and egg replacement that do not have the potential harmful side effects, such as increased risk for heart disease and certain cancers, that current replacement methods pose. Cell-based therapies can be used in post-menopausal women, women who have had ovarian cancer, and women who have experienced damage to their ovaries from other sources.
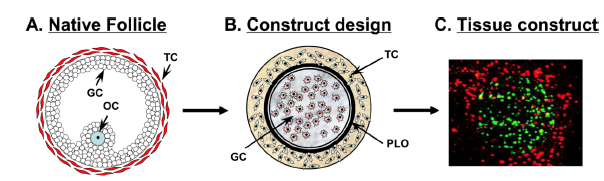
Figure 1. Ovarian follicle construct design.
A) The naturally found antral follicle is the basis of the tissue engineering design for my project.
B) A schematic of the construct that I will be making in the collagen matrix to mimic the native follicular structure.
C) Confocal microscopy that demonstrates the ability to localize the granulosa cells (green) in the middle of the construct surrounded by theca cells (red). [TC= theca cell; GC= granulosa cell; OC= oocyte; PLO= poly-L-ornithine encapsulation]
Ovarian follicles have three major components: the oocyte (gamete) surrounded by layers of granulosa cells and theca cells (hormone production). Several techniques were studied to assay the effects of different methods of culturing on hormone production. So far, the best approach has been mimicking the natural multilayered structure of ovarian follicles ex vivo with granulosa cells surrounded by a layer of theca cells. It has been found that the engineered follicles do indeed secrete sex hormones in a morphologically-dependent manner; the multilayered approach produces more hormone than simply a random mixture of the theca and granulosa cells. With the ability of this therapy to exhibit feedback control over hormone production, it offers the potential benefit of maintaining natural levels of the hormones as opposed to the conventional replacement therapies that offer no great solution to controlling blood hormone levels. This will be an important part of the study when they begin in vivo research.
Currently, we are characterizing a 3D collagen matrix and structure that closely mimics the natural environment within the ovary. My specific role in the project will be to test and define the importance of the ratio of granulosa cells to theca cells as well as find the optimum total number of cells in each follicle construct. I will be analyzing each ratio and follicle size for the ability to produce a physiologically normal level of estrogen and progesterone as well as assessing overall cell viability.
Future Plans:
Ultimately, my career goal is to become a physician, particularly a surgeon. I am pursuing an MD/PhD because of my passion for research. Having the ability to use my skills in a research setting to help people on a much broader scale, in addition to working directly with patients, is the ideal way I want to establish my career in medicine.


