My name is Rajan Choudhary, and I am an undergraduate at the University of Oxford. Currently, I am halfway through my medical degree. As a part of my Bachelor of Arts year, I undertook research at the Botnar Musculoskeletal Research Centre with Dr. David Murray and Dr. Hemant Pandit’s team. My project focused on the Oxford Knee, a unilateral knee replacement (UKR) available as a surgical option for patients with knee osteoarthritis. This implant has been shown to have many advantages over the more traditional total knee replacement, including quicker recovery and lower surgical risks.
In my study, I sought to determine whether the presence of osteophytes (bony spurs forming on the joint border) in the lateral compartment of the knee or horizontal subluxation (movement of the femur away from the midline axis ) of the femur and tibia in patients pre-operatively affects the 10-year outcome of patients with a UKR. I examined the 10-year implant survival and functional outcome of the UKR in 217 patients with and without osteophytes in the lateral compartment, or subluxation, of the knee. The presence of these markers would normally make a patient ineligible for surgery in the eyes of many orthopaedic surgeons. To determine their importance, I compared patient reported outcomes of knee functions at 10 years between groups with and without these markers on X-rays taken pre-operatively. Statistical analysis showed there to be no difference in the 10-year outcome between patients with or without these markers. In conclusion, patients with osteophytes or subluxation are eligible for this surgery. The impact of this study in orthopaedics would be to increase the population of patients that would benefit from the surgery.
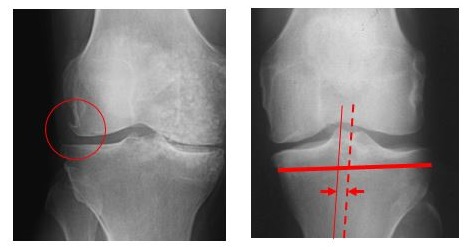
Figure 1. Indications of osteoarthritis in the knee joint.
In the image on the left, an example of an osteophyte, a bony spur, is marked by a circle. In the X-ray on the right, an example of subluxation (horizontal movement of the femur away from the midline axis) of the knee is depicted. The midline is represented by the dashed line, and the position of the femur is represented by the thin line. The subluxation, in this case, is the distance between these two vertical lines, as indicated by the arrows.
Systematic review of T cell immunotherapy for cancer
This summer, I will be working under the guidance of Dr. Andrew Carr, Dr. Marc Feldman, and Mr. James Smith to produce a systematic review on T-cell-based immunotherapy as a treatment option for cancer. Systematic reviews collate research papers published on a particular subject and summarise the findings. Unlike traditional reviews, which tend to have a narrative and are more descriptive, systematic reviews involve a detailed and comprehensive search strategy to remove potential selection bias and may include a statistical component to summarise results from multiple studies into a single quantitative value estimating the progress made in a particular field.
Cancer is a disease of uncontrolled cellular replication, and our current therapeutic options attempt to utilise this fact by targeting sites of the body with high rates of cellular replication through radiation and chemical damage to the cells in order to induce apoptosis. Unfortunately, this “carpet bomb” approach results in collateral damage to non-cancerous regions, best typified by loss of hair. Cellular immunotherapy, on the other hand, aims to use our body’s intrinsic mechanisms for dealing with pathogens to target and remove cancerous cells. T cells are an effector arm of the immune system. Receptors on the surface of T cells are able to recognise specific cell markers with a high degree of accuracy and cause cytotoxic damage only to cells recognised as pathogenic.

Figure 2. Methods of creating receptors targeting tumour markers1
(a) Tumours are obtained from the patient and T cells that have already targeted the tumour are extracted. They are then tested to find the T cell receptor (TCR) best suited for targeting the patient’s tumour. The DNA encoding this TCR is inserted into T cells, and these genetically modified cells are induced to replicate. Finally, the T cells are inserted back into the patient’s blood to begin treatment.
(b) Chimerical antigen receptors (CARs) are constructed from the heads of antibodies, the region capable of recognizing markers. These are genetically engineered to act as receptors capable of starting an immune response in T cells when activated by tumour markers, similar to TCRs. In much the same way as option (a), the genetic material for these chimeric antigen receptors is inserted into T cells and expanded before being given to the patient.
Efforts have been made to identify, isolate, and increase populations of T cells capable of recognising cancerous cells. The resulting product can be given to the patient as a therapy to target cancer. Recently scientists have been able to produce genetically modified T cell receptors that are capable of recognising these surface markers with greater success. Two main strategies exist (Figure 1): genetically modified T cell receptors that resemble native T cell receptors found on T lymphocytes and also Chimeric Antigen Receptors (CARs), which have structural similarity to antibodies produced by B cells. Currently, such strategies do have limitations, including off-target effects. My systematic review will provide an objective measurement of how far research has progressed. This report will help us understand the current progress of the field, not only the advances and benefits offered by this therapy but also the pitfalls and side effects reported by clinical trials as well.
Future Plans:
I plan to continue my clinical training for the next three years, after which I will become a junior doctor working for the National Health Service. Currently, my plans for specialising are unknown, as I feel it is better to gain broad experience before making this decision. However, I am interested in multiple areas, including neuroscience and aerospace physiology.
References:
1. Restifo, N.P., Dudley, M.E. & Rosenberg, S.A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12, 269-281 (2012).


