My degree and experience in the processing and manufacturing of biological systems for therapeutic purposes have greatly influenced my passion for regenerative medicine. I have completed a Bachelors of Engineering in Biochemical Engineering at University College London (UCL) where I will be returning to complete the final year of my Masters of Engineering in Bioprocess Management later this year. I am particularly interested in the development and application of biological processes for the treatment and prevention of disease. As a Project Engineer in the Technical Manufacturing Support team at GlaxoSmithKline, I worked on a number of process improvement projects. One of them was the installation and validation of process equipment which contributed to the timely production of Augmentin for global supply, in accordance with regulatory requirements. The plant, which abided by current Good Manufacturing Practices, showed me the types of regulatory challenges, which often inhibit the onset of technological and therapeutic progress.
Joining Prof. Andrea Streit’s research team during a summer internship at the Craniofacial Development and Stem Cell Biology Department at King’s College in London, further strengthened my understanding of the connection between research and clinical applications. Developmental programming exists in the genome, initially, in the form of coding and non-coding genes. Throughout development, specific genes are activated or repressed by various transcription factors which contribute to cellular differentiation. Although the specific components that govern these events have been identified, Prof. Streit realized the importance of defining the Gene Regulatory Networks (GRNs) in order to establish predictive differentiating models during normal development and disease. GRNs offer a systematic explanation of the developmental process, organogenesis and cellular differentiation and thus provide a representation of the cell fate decisions at molecular level.
During my internship, I assisted with the electroporation of small interference DNA at different stages of the chick embryo, a well-understood model system. This helped to evaluate the transcriptional factors and receptors influencing the network components during development. Prof. Streit’s lab devised an experimental workflow describing how to construct a GRN using the chick embryo as a model. The findings on the nature of cellular development as a result of the gene regulatory network were published in the article: “Experimental approaches for gene regulatory network construction: The chick as a model system” Streit A. et al, 2012.
This experience has brought to my attention the importance of bridging the gap between academic research and the application and commercialization of ideas as well as some as the challenges commonly faced by innovators en route to clinical progress. This is essential, particularly in the fast-moving field of regenerative medicine, and is one of the many characteristics of working in the Karp Lab that appeal to me. I am very passionate about the development of research concepts into therapeutic or technological products, particularly the evaluation and translation of an idea into its viable medical potential.
I will continue to foster my interest in regenerative medicine while I work under the guidance of Dr. Jeff Karp and Executive Director Brock Reeve at the Harvard Stem Cell Institute. The Karp Lab has established a multi-disciplinary environment to approach contemporary medical challenges, which has created a successful platform for numerous technologies. I feel both inspired by Dr. Karp’s work and motivated by SENS Research Foundation’s broad-based research to contribute to progress in the field of regenerative medicine.
Cell therapy has been heavily explored in recent years for its therapeutic potential. Increased attention has been given to naturally occurring cellular by-products, extracellular vesicles (EVs), which have been identified to play an important role in cellular communication, and the overall healing process. My project will evaluate the therapeutic potential of extracellular vesicles against established and developing therapies, in particular cell therapy, in order to yield a comparable and informative appraisal to guide research and clinical endeavors.
Systematic Review of the Therapeutic Potential of Extracellular Vesicles (EVs)
Research exploring the potential of EVs as therapeutic agents has exponentially increased in recent years, giving rise to a wide range of unsystematic and uncomparable results. During my time working for SRF at the Harvard Stem Cell Institute and the Karp Lab, I hope to compile a systematic review and meta-analysis of such studies to objectively evaluate the therapeutic potential of EVs. This report will attempt to normalize EV therapy such that researchers can compare it to the efficacy of existing and equivalent therapeutics, particularly cell-based ones.
Intercellular communication is an essential part of cellular development. Eukaryotic cells are able to produce and release membrane-derived vesicles to reach both neighboring and distant cells. These extracellular vesicles contain proteins, lipids, and nucleic acids and play an important role in the maintenance of stem cells, tissue repairs, and immune surveillance as well as a series of other underlying processes in the body. As a result, EVs have been increasingly evaluated for their potential as therapeutic agents in the treatment of such health problems as cerebrovascular and myocardial conditions, vascular diseases, pulmonary diseases, immune diseases, traumas and musculoskeletal disorders.
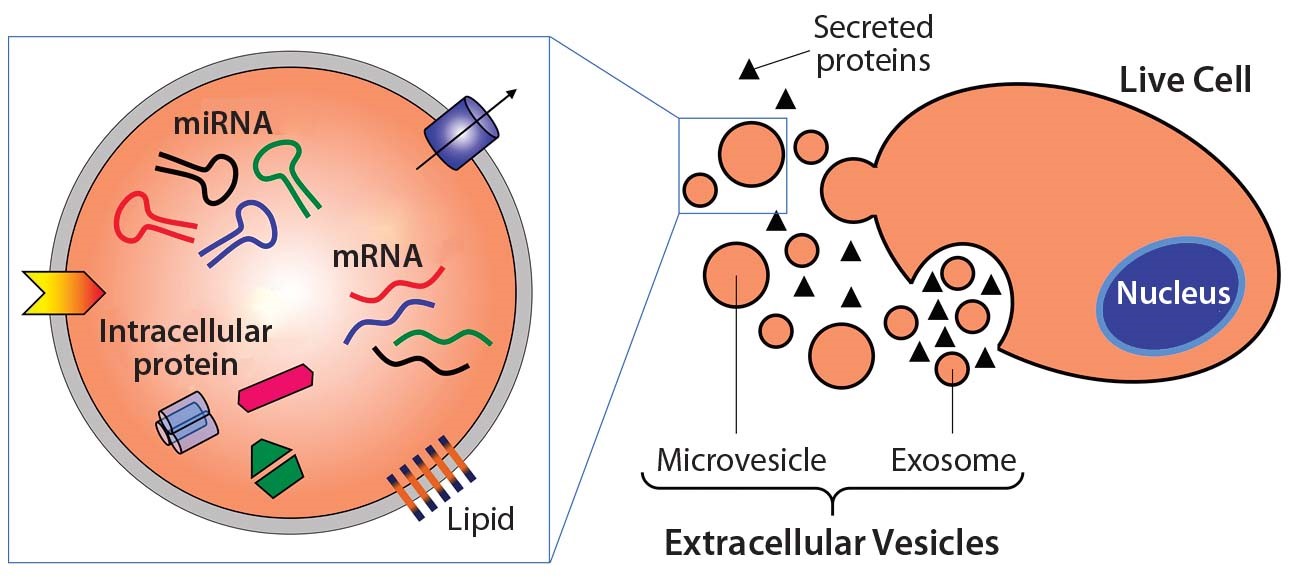
Figure 1. Extracellular vesicle formation and composition[3].
Eukaryotic cells give rise to EVs through a variety of ways: ectosomes and microparticles are produced via direct membrane budding while exosomes are released via internal multivesicular components. Although this can give rise to membrane vesicles of different sizes, they typically functionally resemble the parent cell. Microvesicles can exist in the range of 50 – 1000nm, while exosomes are much smaller in the range of 40 – 120nm [2]. Figure 1 shows an example of common EV contents and displays the process by which EVs are commonly released from the cell.
The outcome of this report will help to define the expectations and potential of EV therapeutics. I hope that through reconciling heterogeneity in EV research, results will become more comparable and informative and help guide this emerging field towards feasible endpoints.
Future Plans:
I am interested in developing my understanding of the body’s adaptability to disease and functional defects in order to identify ways in which we could replicate the process through regenerative medicine. I aim to remain deeply involved in the development and commercialization of clinical therapeutics, where I can contribute to the translation of scientific discoveries into viable medical applications.
References:
[1] “Experimental approaches for gene regulatory network construction: The chick as a model system”, Streit A., Tambalo M., Chen J., Grocott T., Anwar M., Sosinsky A., Stern C. D., 2012.
[2] “Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials”, Vlassov A., Magdaleno S., Setterquist R., Conrad R., 2012.
[3] “Extracellular Vesicles Commercial Potential As Byproducts of Cell Manufacturing for Research and Therapeutic Use”, Smith J. A., Ng K. S., Mean B. E., Dopson S., Reeve B., Edwards J., Wood M. J. A., Carr A. J., Bure K., Karp J. M., Brindley D. A., 2015.


