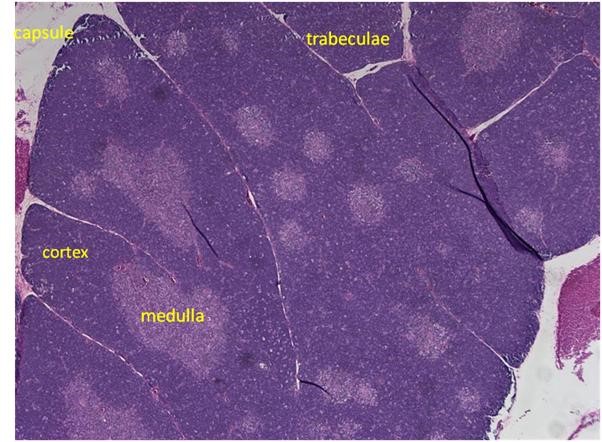
Figure 1. H&E staining of a rat thymus.
Creation of thymus tissue scaffold
Different hydrogels made of either a fibrin/collagen gel or a hyaluronic acid/fibrin gel were tested for strength and ability to maintain cells. These gels were chosen because they mimic wound-healing processes and are easily created and modified. All scaffold types were seeded with thymic epithelial cells and bone marrow cells and then cultured in vitro for 21 days. The bone marrow cells provide growth factors that are needed for T-cell production. The cells used for reseeding were obtained from a normal mouse thymus and were expanded in vitro. In order to maintain their characteristics, the cells were cultured with a pig native thymus powder that was been gamma irradiated in order to maintain sterility. The powder mimics the environment of the thymus and provides factors that allow the cells to proliferate.
After 21 days, the scaffolds were collected and tested for T-cell activity using flow cytometry. Thymus markers CD3, CD4, and CD8 were assayed to verify T-cell maturation. The scaffolds were also sectioned and checked for CD3, CD4, and CD8 markers using immunohistochemical staining.
Testing hydrogel scaffolds
Initial data for a hyaluronic acid hydrogel showed degradation after three weeks. However, the gel did support healthy growth of the cell types. After the first week, cell retrieval from the gel was attempted using collagenase/hyaluronicase solution. This resulted in two problems. The first problem was that the gel had begun to degrade and was hard to retrieve in one full piece. The gels were also not viscous enough to be sectioned. In addition, the enzyme solution was too strong and very few viable cells were retrieved. This process was repeated and shorter incubation periods for the enzyme were tested. Again, very few cells were retrieved. This made flow cytometry analysis impossible. In order to increase the strength of the gel, the hyaluronic gel was mixed with a small concentration of fibrin and cultured with aprotinin to try to decrease degradation. The fibrin was added to create a tighter matrix between the components of the hyaluronic acid. Aprotinin was used because it blocks enzymes that are released from serum in the media that is used for culture. These enzymes can cause the gel to degrade and release toxins that can be detrimental to cell growth. In addition, a fibrin collagen gel was tested.
After a three-week period, the fibrin collagen gel was easily retrieved from a 48-well plate, indicating a strong matrix. The retrieval of the gel was repeatable because there were multiple independent successes within the plate. The hyaluronic acid gel with fibrin was sturdier than the initial hyaluronic gel, but still fractured upon retrieval, indicating that the fibrin collagen gel was sturdier. The results also showed that the aprotinin was successful at preventing degradation. However, as a consequence, flow cytometry could not be performed because the enzymes used for gel degradation were not successful in breaking down the fibrin-based gels. Furthermore, although the gels appeared sturdy, when they were placed in optimal cutting temperature (OCT) compound for frozen sectioning and immunostaining, ice crystals formed within the pores of the hydrogels, causing the gels to not section fully and break apart. When immunostaining was performed on the sectioned gels, the staining was not specific. Significant auto-fluorescence from the gels produced inconclusive results.
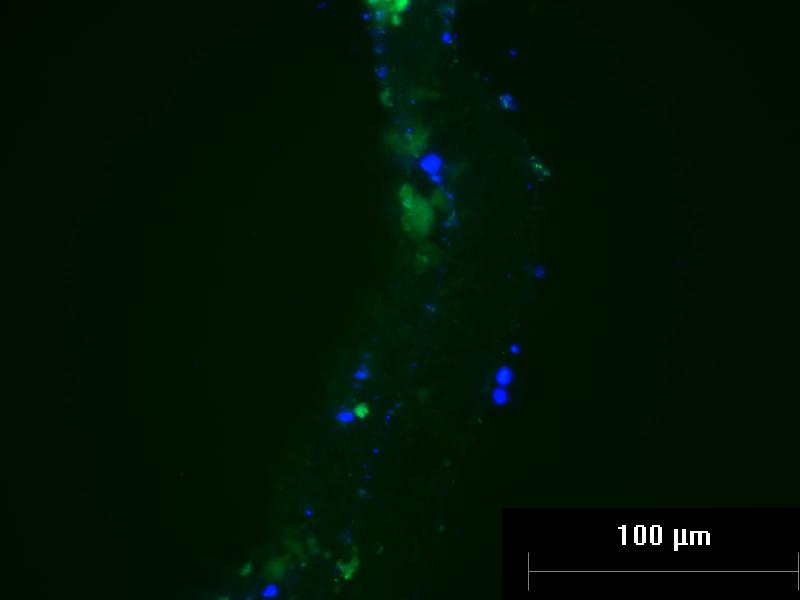
Figure 2. Auto-fluorescence of Thymus Hydrogel Scaffolds.
Fibrin collagen gel seeded with mouse thymus epithelial cells and bone marrow cells was stained for CD3 after 3 weeks. Green auto-fluorescence obscured CD3-specific staining. Cells within the hydrogel are marked with DAPI-staining (blue).
Conclusion and Future Directions
The results of my project led to the conclusion that the fibrin collagen gel is the strongest, and future research should focus on this type of gel because it did not break apart when taken out of the 48-well plate. This could make it easy to implant in an in vivo study. However, a way to break down the gel to retrieve the cells to test for T-cell production needs to be established. Plasmin should be tested because it is known to cleave bonds between fibrin during wound healing and other processes. A method for protecting the gel during sectioning should be researched as well. The use of plastic resins or dehydration techniques have been suggested for histological analysis of hydrogels4. Also, creating aggregates of cells and then placing them within the gel has the potential to promote T-cell production and should be tested. This is because the cells rely on signals from each other to create T-cells, and if they are just suspended in a gel they are not in close enough proximity to make cell-to cell contact and produce the necessary factors.
References:
1. Seiki, K., & Sakabe, K. (1997). Sex hormones and the thymus in relation to thymocyte proliferation and maturation. Archives of histology and cytology,60(1), 29-38.
2. Vianello, F., & Poznansky, M. C. (2007). Generation of a tissue-engineered thymic organoid. In Immunological Tolerance (pp. 163-170). Humana Press.
3. Lee, K. Y., & Mooney, D. J. (2001). Hydrogels for tissue engineering. Chemical reviews, 101(7), 1869-1880.
4. James, R., Jenkins, L., Ellis, S. E., & Burg, K. J. (2004). Histological processing of hydrogel scaffolds for tissue-engineering applications. Journal of Histotechnology, 27(2), 133-139.


