My name is Sebastian Aguiar, and I graduated in May 2014 from Pitzer College, Claremont where I majored in Human Biology and minored in History. I conducted my thesis research at the Icahn School of Medicine at Mount Sinai in New York in the lab of Dr. John Morrison. The project was entitled “Characterizing a novel monoclonal AMPA receptor 1/2/3 antibody in the hippocampus and prefrontal cortex of rat, monkey, and human.” My work with Dr. Morrison employed immunohistochemistry, confocal laser scanning microscopy, and electron microscopy to validate a new antibody used for visualizing different permutations of AMPA receptors in the brain. AMPA receptors are of great medical interest because they are the most populous excitatory ionotropic receptor in the central nervous system, they mediate learning and memory, and they are critical to epilepsy and dementia. Researchers are investigating a class of drugs known as ampakines that augment AMPA receptor function and have proven promising in the treatment of cognitive disorders.
Not all antibodies are created equal and must be proven to bind exclusively to the target. My thesis project in the Morrison lab showed that the antibody co-localized with another protein found in the postsynaptic density, a region that receives neurotransmitter input from the preceding neuron. In other words, the antibody was locating the AMPA receptors where they should be found. With further validation, this tool, licensed to the company Millipore, will enable researchers to map the distribution of different types of AMPA receptors throughout the nervous system.
One of my favorite courses at college was Neuropharmacology. I enjoyed it so much that the professor and I co-authored an article in the peer reviewed journal Rejuvenation Research reviewing the nootropic herb Bacopa monnieri, a promising therapy for neurodegeneration. My interest in the way drug molecules interact with the brain led me to pursue this area further. I even won third place in the Henry T. Kravis Concept Plan Competition at the Drucker School of Management for a pharmaceutical business proposal for a low-toxicity cognitive enhancing supplement that included Bacopa compounds blended with nine other natural products.
At Claremont I was also involved in various extracurricular activities, such as serving as the Vice-President of the Student Senate of Pitzer College. I was also a member of the Model UN, the Claremont Colleges Debate Union, the Student Investment Committee, and the Trustee Endowment Committee.
I have been following the SENS Research Foundation (SRF) since my larval stages in high school. SRF has crusaded valiantly for what I take to be, from a demographic and utilitarian perspective, the most important goal of medical science: the attenuation of the massive suffering concomitant with aging. Physiological aging is unlike any other condition in that we all suffer from it. Cardiovascular disease, dementia, stroke, diabetes, cancer — the primary but overlooked risk factor is age. Even if science outright cured one of these diseases, another would be right around the corner with an exponentially increasing probability of striking. Reducing the rate of aging addresses a litany of other conditions that until recently have been regarded as separate. Aging research provides the most efficient tactic for engineering an extended human healthspan. This summer, I am studying the role of neuropeptides in C. elegans longevity under the mentorship of Dr. Jennifer Garrison at the Buck Institute for Research on Aging.
Neuropeptide Signaling and Longevity in C. elegans
Hormones are secreted factors that act at a distance from their originating cell. Many hormones are produced by specialized endocrine cells. One example of this type of hormone is insulin, which is produced by the pancreatic beta cells in the Islets of Langerhans and which work by binding to receptors on the external membrane of cells throughout the body to increase glucose uptake from the bloodstream. Insulin dysfunction results in metabolic syndrome, obesity, and diabetes.
Other hormones, known as neuropeptides, are processed and secreted exclusively by neurons. These peptides act as neuromodulators, meaning they do not rewire the neural network. Instead, they affect neural output by altering more subtle characteristics, such as the response threshold of the target neurons. Approximately 90 neuropeptides are known to function in the mammalian brain. A neuropeptide can broadcast a signal across a larger distance and function for a longer timescale than classical small molecule neurotransmitters like serotonin, dopamine, norepinephrine, glutamate, and GABA. Examples of neuropeptides include: neuropeptide Y and orexin, which are involved in appetite and mood; substance P, enkephalin, and dynorphin, which are involved in pain perception; and neurotrophins like brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), which are involved in neural survival, neuroplasticity and cognition.
There is good reason to believe peptides are critical to aging. Although they are not neuropeptides, insulin and insulin-like growth factor 1 are central to metabolic aging.1 Gonadotropin-releasing hormone (GnRH), a neuropeptide released from the hypothalamus, has been shown to regulate inflammation-related aging. GnRH is classically understood as a regulator of reproduction. However, Zhang et al. showed that GnRH is negatively controlled by two inflammatory proteins, NF-kB and IKK-B. Inhibiting these proteins augmented GnRH expression, and the administration of GnRH attenuated aging-impaired neurogenesis (particularly in the hippocampus and hypothalamus).2 This evidence establishes a powerful case for neuropeptides in aging.
Recent evidence shows that the neuropeptides substance P and calcitonin gene-related peptide (CGRP) play a role in longevity via nociception (pain perception). Organisms in chronic pain live shorter lives. Knocking out or blocking a receptor (TRPV1) that leads to substance P and CGRP release results in augmented lifespan and an improved metabolic profile in mice. TRPV1 is also responsive to capsaicin, a compound found in spicy foods. It appears that CGRP antagonizes insulin release from pancreatic beta cells.3
Two analogous but functionally distinct neuropeptides of nascent interest to aging researchers are vasopressin and oxytocin. Made by magnocellular neurosecretory cells in the human posterior pituitary gland, they are conserved across higher species. Oxytocin plays a key role in social cognition, pair bonding, reproductive physiology, inflammation4 and wound healing.5 However, recent evidence suggests an additional role for oxytocin in muscle tissue maintenance and regeneration. It is known that the plasma concentration of oxytocin declines with age, and it has been recently shown that oxytocin knockout mice display premature sarcopenia and that systemic oxytocin administration enhances aged muscle stem cell proliferation via the MAPK/ERK signaling pathway.6
The Garrison lab plans to explore the possible role that a handful of candidate neuropeptides play in C. elegans tissue degeneration and longevity. The nematode worm Caenorhabditis elegans is a celebrated model organism popularized by Nobel laureate Sydney Brenner. The worm possesses qualities that render it a premier model for development, neuroscience, and aging research.7
Figure 1. C. elegans neural connectivity diagram.
This diagram, called a connectome, depicts the complete wiring of the 302 neurons found in an adult C. elegans worm. Neuropeptides affect the survival, growth, and differentiation of these neurons as well as enable more complex dialogue between neurons.
Hamilton et al. (2005) conducted a systematic RNAi screen for longevity genes in C. elegans, implicating a number of proteins involved in neuropeptide processing (for example, the carboxypeptidase egl-3, which resulted in life extension).8 Given the evidence that neuropeptides modulate aging, the goal of my summer research at the Garrison lab will be to ascertain the longevity of various neuropeptide overexpressing and knockout worms.
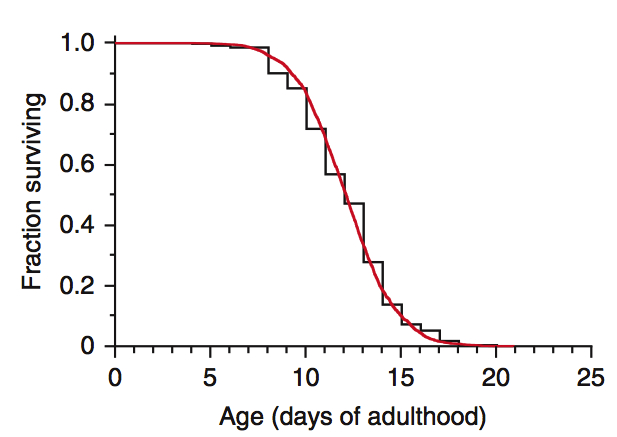
Figure 2. Kaplan-Meier Survival Curve showing organismal longevity over time.
Pictured is a sample of the type of longevity data I will be collecting during the course of my project. The data are reported typically as mean and maximum lifespan.
Future Plans:
In the future, I will be applying to graduate school to study aging with a molecular biology or neuroscience emphasis. I am particularly interested in neuropharmacology, natural products chemistry, and cognitive aging. Prior to pursuing my graduate studies, I plan to join a laboratory as a research assistant to continue to pursue these interests.
References:
1. Wolkow CA, Kimura KD, Lee MS, Ruvkun G (2002). Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 290(5489):147-50.
2. Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li Bo, Liu G, Cai D (2013) Hypothalamic programming of systemic ageing involving IKK-b, NF-kB and GnRH. Nature 497, 211–216.
3. Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, de Magalhaes Filho CD, Merkwirth C, Dillin A. (2014) TRPV1 Pain Receptors Regulate Longevity and Metabolism by Neuropeptide Signaling. Cell 157, 1032-1036.
4. Szeto A, Rossetti MA, Mendez AJ, Noller CM, Herderick EE, Gonzales JA, Schneiderman N, McCabe PM. (2013). Oxytocin administration attenuates atherosclerosis and inflammation in Watanabe Heritable Hyperlipidemic rabbits. Psychoneuroendocrinology 38(5), 685-693).
5. Gouin JP, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK. (2010). Marital Behavior, Oxytocin, Vasopressin, and Wound Healing. Psychoneuroendocrinology 35(7): 1082–1090.
6. Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM. (2014) Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nature Communications 5, 4082.
7. Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. (1993) A C. elegans mutant that lives twice as long as wild type. Nature 464, 504-512.
8. Hamilton B, Dong Y, Shindo M, Liu W, Odell I, Ruvkun G and Lee SS. (2005) A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 19(13):1544-1555


