Sean is a senior at the Massachusetts Institute of Technology (MIT), where he is studying computer science and biology. His long-standing interest in neurology led him to MIT’s Synthetic Neurobiology Group, led by Dr. Edward Boyden. As part of an interdisciplinary team of electrical engineers and molecular biologists, he is studying the effects of emotions such as fear on learning and memory in mice.
His interest in bridging the gap between basic science and clinical medicine led him to the 2013 SENS Research Foundation (SRF) Summer Internship Program. As a member of the Buck Institute for Research on Aging’s Dr. Julie Anderson, Sean studied the effects of lithium on a mouse Parkinson disease model. This research built upon previous research which suggested that lithium, an established treatment for bipolar disorder, may also be effective at treating Parkinson’s. Sean’s summer project investigated the effect of lithium in a Parkin transgenic mouse model.
Investigating the Mechanism of Lithium Treatment of a Parkinson’s Disease Model
Previous research in Dr. Anderson’s laboratory revealed that lithium, a drug commonly used to treat bipolar disorder, also may prevent neurodegeneration in an animal model of Parkinson’s disease. Working with postdoctoral researcher Dr. Christopher Lieu, I tried to determine what molecular pathway is associated with the previously observed effects of lithium on the symptoms of a Parkinson’s disease animal model.
Two possible mechanisms were investigated: autophagy (the process by which a cell can recycle and remove metabolic cellular debris, protein aggregates, and damaged organelles) and inflammation. One theory argues that dysfunction in the neurocellular autophagy pathway is responsible for the neuronal degeneration and resulting loss of motor control observed in Parkinson’s disease. If so, reactivation of the autophagy mechanism may be responsible for the neuroprotective properties of lithium in a Parkinson’s disease model. We also tested the possibility that the neuroprotective properties of lithium may be a result of lithium’s effect on neuronal inflammation.
Autophagy and inflammation markers were measured in four different groups of mice: untreated wild-type (non-Parkinson) mice, lithium-treated wild-type mice, untreated Parkin mice, and lithium-treated Parkin mice. Although analysis of autophagy markers have thus far been inconclusive, preliminary results indicate lithium may have an effect on neuronal inflammation. High levels of a protein called GFAP (glial fibrillary acidic protein) is correlated with inflammation in the brain. Lithium treatment appeared to decrease the level of GFAP detected by antibody staining in striatal astrocytes of Parkin mice. However, a similar effect was not observed in the cortex. Lithium also appeared to increase GFAP levels in non-Parkin control mice. Further studies will be needed to verify these results as well as determine the contribution of the autophagy pathway to the neuroprotective properties of lithium.
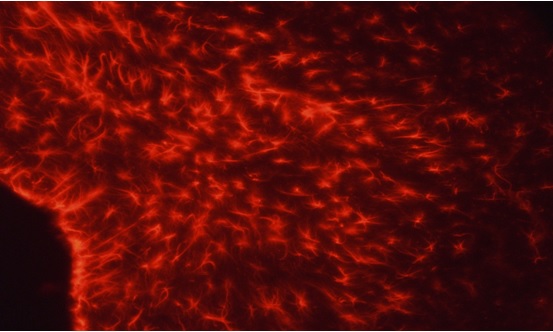
Figure 1. Representative image of GFAP localization (red) in striatal astrocytes.
Future Plans:
Sean has returned to MIT for his senior year where he will continue to conduct research under the supervision of Dr. Edward Boyden and postdoctoral fellow Dr. Annabelle Singer in the Synthetic Neurobiology Group at MIT. When he graduates, Sean plans to pursue a career as a neural engineering developing technology that will alleviate neurological and neuropsychiatric damage.


