Meredith Giblin is a senior at Rensselaer Polytechnic Institute (RPI) in Troy, NY, where she is majoring in Biochemistry and Biophysics. During her first three years at RPI, Meredith worked in the laboratories of Dr. Robert Linhardt and Dr. Patrick Maxwell. While in the Linhardt lab, she optimized an endotoxin purification protocol as part of a project to study the enzymatic modification of the glycan chain of the protein syndecan 1. Her ongoing work in the Maxwell lab is exploring the effects of retrotransposons on the aging process in yeast.
Meredith spent the summer working in the lab of Dr. Judith Campisi at the Buck Institute for Research on Aging with postdoctoral fellow Dr. Christopher Wiley. Her summer project focused on the role of histone deacetylases in inducing the senescmence-associated secretory phenotype (SASP), which consists of a myriad of factors secreted by senescent cells that are associated with inflammation and malignancy.
A Study of the Effect of Histone Acetylation on ATM Activation and the SASP
“Cellular senescence is a process in which a cell ceases to proliferate in response to oncogenic stimuli. Ironically, although senescence helps protect the cell in question from becoming cancerous, the associated SASP has been shown to contribute to age-related diseases, in particular cancer. The Campisi lab has previously demonstrated that several proteins involved in the DNA damage response (DDR) pathway are also necessary for the SASP. Inhibition of histone deacetylases (HDACs) and activation of the ataxia telangiectasia mutated (ATM) protein in turn activate the SASP. This suggests that the state of the chromatin rather than the physical breaks in DNA is responsible for initiating the SASP response in senescent cells. My project sought to characterize the role specific HDACs play in ATM activation and SASP induction.
To determine which HDACs play a role in the SASP, I knocked down the level of specific HDACs using small hairpin RNAs. The effect of the HDAC knockdown on the SASP was determined by measuring the level of a well-characterized SASP factor, interleukin-6 (IL-6). Prior studies indicated that knockdown of relevant HDACs would trigger a SASP response. In addition, ATM activation was assessed via Western blot analysis of a protein downstream of ATM, called p-AMPK. Detection of p-AMPK would indicate ATM activation.
The following figure summarizes the results of the IL-6 study. Note that loss of HDAC1 function led a three-fold increase in IL-6 levels. Surprisingly, knockdown of some HDACs resulted in both apparent activation of ATM simultaneously with no little or no increase in IL-6 levels. Although this appears to be a discrepancy in the model, other mechanisms in the cell may be leading to AMPK phosphorylation. Further studies of other proteins downstream of ATM, such as p-CHK2 or p-53 S15 could clarify these results.
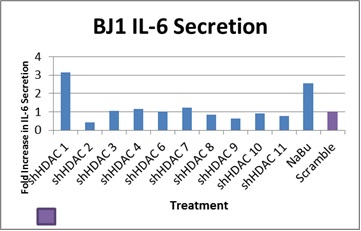
Figure 1.
Analysis of ATM and HDAC5 provided further evidence that IL-6 secretion is ATM-dependent. As depicted in Figure 2, knockdown of HDAC5 resulted in increased IL-6 levels. However, knockdown of both HDAC5 and ATM produced a ten-fold decrease in IL-6 levels. To further more comprehensively investigate the effect of HDAC5 and other HDACs on the SASP, further SASP markers will be evaluated in the future.”
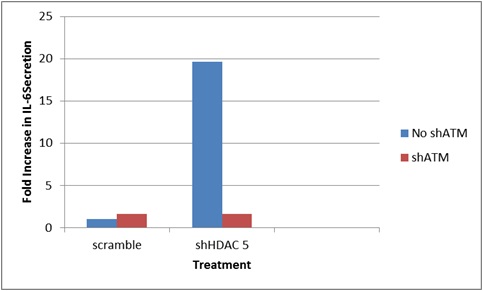
Figure 2.
Future Plans:
Meredith is currently completing her final year at RPI, where she continues her studies of the effect of retrotransposons on genome stability in yeast. After graduation, she plans to pursue a Ph.D. in molecular genetics, studying the genetics of neurodegenerative disorders such as Alzheimer’s disease.


